Abatacept Shows Promise for RA-ILD Save
Abatacept (Orencia) may be a useful treatment option for patients with rheumatoid arthritis (RA) who develop interstitial lung disease (ILD), a Spanish study suggested.
Among 63 patients with RA-ILD, after a mean follow-up time of 9.4 months, two-thirds of patients remained stable on the Modified Medical Research Council (MMRC) dyspnea scale, and an additional one-quarter showed improvement of at least one point on the MMRC 0-4 scale, according to Miguel A. González-Gay, MD, of the University of Cantabria in Santander, and colleagues.
In contrast, only one of 19 patients who were asymptomatic at baseline developed grade 1 (mild) dyspnea at that time point, the researchers reported in Seminars in Arthritis & Rheumatism.
The prevalence of RA-ILD has been estimated at up to 44%, and is the second leading cause of death among patients with RA, behind cardiovascular disease. Risk factors that have been identified for ILD among RA patients include older age, male sex, and smoking.
Management presents particular challenges, in that a number of the common RA treatments have been linked with exacerbations of ILD, including methotrexate and anti-tumor necrosis factor agents.
Blockade of T-cell co-stimulation in a murine model of hypersensitivity pneumonitis with a chimeric fusion protein has resulted in the inhibition of pulmonary inflammation, but it has not been known whether blocking T-lymphocyte co-stimulation in RA patients with abatacept could influence ILD.
To explore this possibility, González-Gay and colleagues conducted an open-label registry study that included patients from 31 Spanish centers who had at least 3 months of exposure to abatacept from 2000 to 2016.
Of the 63 patients found to have ILD, 36 were women. Mean age at the time of starting abatacept was 63, and the mean durations of RA and ILD were 6.8 years and 1 year, respectively. Most patients were positive for rheumatoid factor or cyclic citrullinated peptide antibodies, and more than 60% were current or former smokers.
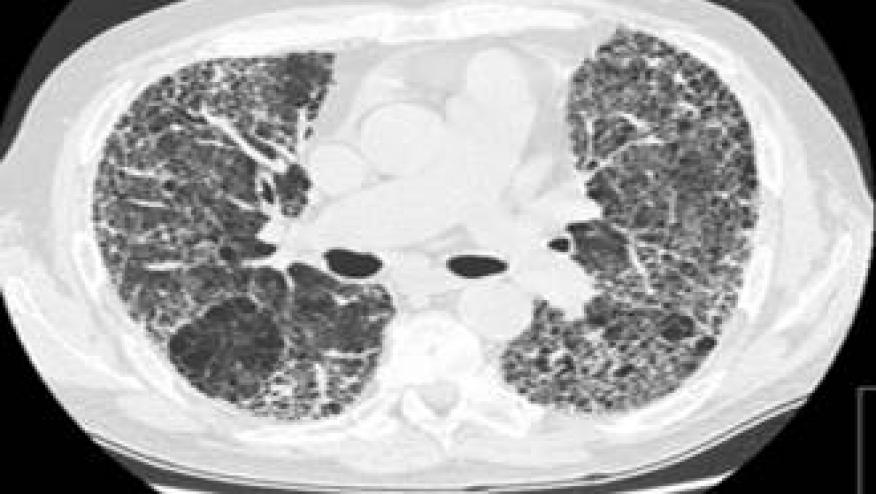
The patterns of ILD were usual interstitial pneumonia in 43% of patients, nonspecific interstitial pneumonia in 27%, and other patterns such as bronchiolitis obliterans or organized pneumonia in 27%.
Among the medications patients were using at the time of ILD diagnosis were methotrexate in 38, leflunomide in 15, hydroxychloroquine in seven, etanercept (Enbrel) in seven, adalimumab (Humira) in three, infliximab (Remicade) in two, and certolizumab pegol (Cimzia) in two.
The median prednisone dosage was 10 mg/day. Abatacept was given in standard dosages for RA, either 10 mg/kg intravenously every 4 weeks or subcutaneously 125 mg/week. In 26 patients, abatacept was used as monotherapy, while in the remainder, it was given with conventional disease-modifying antirheumatic drugs such as leflunomide, methotrexate, or hydroxychloroquine.
At baseline, most patients had MMRC dyspnea grades of 0, 1, or 2 (asymptomatic, mild, or mild-moderate). Mean forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO) were 87.1 and 64.4% predicted, respectively.
At 12 months, FVC remained stable in two-thirds of patients and improved at least 10% from baseline in almost 20% of patients, while DLCO also remained stable in two-thirds and had a 10% improvement in one-quarter of patients.
Mean disease activity scores in 28 joints declined from 5.03 at baseline to 3.34 at 6 months and 3.51 at 12 months. Median prednisone doses declined from 10 mg/day at baseline to 5 mg/day at month 12.
In 22 patients who remained symptomatic and had high-resolution CT scans performed at month 12, stabilization of ILD was demonstrated in 11 patients, improvement in eight, and worsening in three.
Abatacept was discontinued in 11 patients during follow-up. The reasons included adverse effects such as respiratory tract infections in seven patients, a lack of efficacy for rheumatic symptoms in three, and pulmonary worsening in one. One patient died during follow-up from ischemic heart disease, and another died from an ILD flare after stopping abatacept.
Two potential mechanisms have been proposed to explain the development of ILD in patients with RA, the researchers explained. One theory posits that the immune response against citrullinated peptides in the joint subsequently shifts to the lung, while the other suggests that an idiopathic lung fibrosis triggers the immune response toward citrullinated proteins that provokes the joint inflammation.
Asked to comment on the study, Eric Matteson, MD, a rheumatologist at the Mayo Clinic in Rochester, MN, said that the study "certainly suggests the possibility that abatacept may influence the course of RA-ILD."
Still, he said, that is not conclusive -- "the main reason is that most patients with RA-ILD remain stable, as was the case here."
Matteson also pointed out that the MMRC is a subjective assessment of dyspnea: "It would be important to know the magnitude of change, how many patients moved from one category [of the MMRC] to another, and what the ceiling effects might have been. Longer-term, randomized studies of interventions for RA-ILD are needed."







If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.