Biosimilar SB4 Performs Equally to Etanercept Save
Biosimilars are rapidly growing. Many of these will have their first introduction to countries outside of the USA. There is concern whether biosimilars will provide identical pharmacodynamics, biological function, efficacy and toxicity to reference products, both in the short and long term.
Emery and colleagues have reported the results of a phase III, randomized, double-blind, parallel-group study was conducted at 73 centers across 10 countries in Europe, Latin America and Asia with a goal to compare the efficacy and safety of SB4 (an etanercept biosimilar) with currently marketed etanercept (the reference product).
SB4 is produced by recombinant DNA technology in a Chinese hamster ovary mammalian cell expression system. Similar structural, physicochemical, pharmacodynamic and biological activities of SB4 and ETN have been shown using state-of-the-art analytical methods including peptide mapping, TNF-α binding assay and TNF-α neutralisation cell-based assay and pharmacokinetic studies.
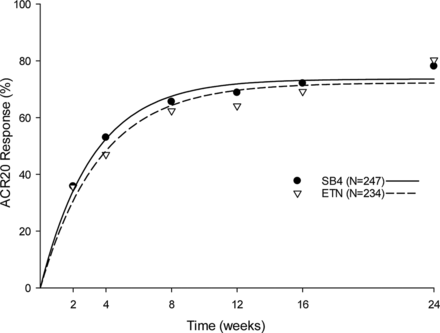
In this study of 596 patients with moderate to severe RA (also treated with or without methotrexate) the ACR20 response rate at week 24 was 78.1% for SB4 and 80.3% for ETN (see figure below). The ACR50 and ACR70 response rates at week 24 were equivalent between SB4 and ETN as well. Treatment-emergent adverse events were noted in 165 (55.2%) of the SB4 patients and 173 (58.2%) of the ETN patients. The authors concluded that the safety profile of SB4 was comparable with that of ETN and was similar to those observed in the pivotal trials with ETN. SB4 showed low immunogenicity profile and good tolerability in this study.






If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.