Hepatotoxicity and TNF Inhibitors Save
Hepatic enzyme elevations with TNF inhibitor use is not uncommon. Minor elevations of AST or ALT in the ATTRACT (RA) and ACCENT I (Crohn’s) trials were seen in 29-42% of patients but extreme elevations (>5 fold) were rare. CORRONA analyzed 6861 RA patients starting TNF inhibitors and found enzyme elevations greater than one times higher than the upper limit of normal (>1x ULN) in 5.9% of AST/ALT determinations, but only 0.77% had >2x ULN elevations (1).
The product labels from most of the marketed TNF inhibitors state that hepatic enzyme elevation may occur, but most patients showing elevated hepatic enzymes are asymptomatic. There are reports of severe hepatic reactions, acute liver failure, > 5 fold LFT elevations, hyperbilirubinemia and liver transplantation with TNF inhibitor use. The product label includes post-marketing reports of severe hepatic reactions, acute liver failure, jaundice, hepatitis and cholestasis.
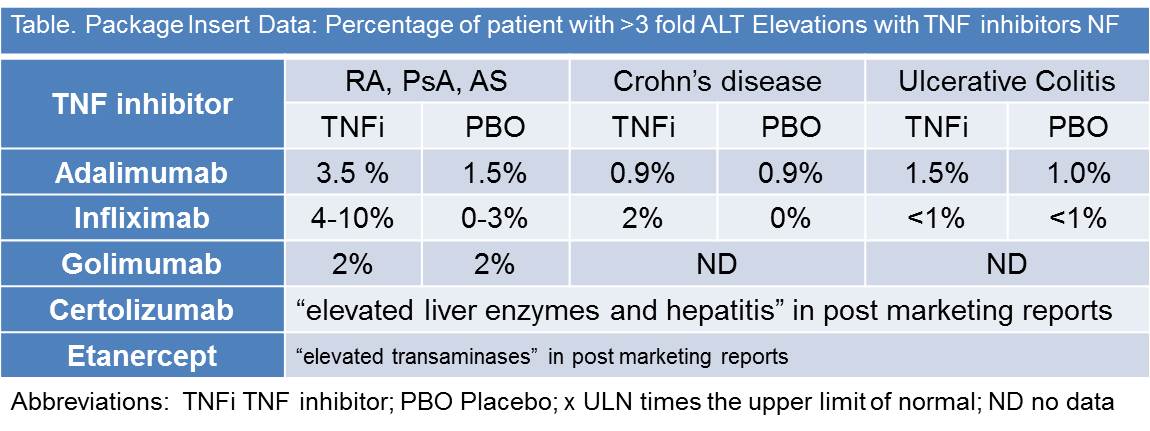 In 2003, the FDA arthritis advisory committee meeting reviewed the rare but serious findings of hepatotoxicity associated with TNF inhibitor use (2). They reviewed 134 reports of liver failure captured from the FDA adverse event reporting system (AERS). A detailed analysis of 50 cases showed 31 were due to infliximab and 19 etanercept. When other potential confounders (e.g., hepatitis, alcohol, MTX, hepatotoxic agents, GVHD) are excluded, 7 of 50 (14%) cases pointed to the TNF inhibitor as the lone offending agent.
In 2003, the FDA arthritis advisory committee meeting reviewed the rare but serious findings of hepatotoxicity associated with TNF inhibitor use (2). They reviewed 134 reports of liver failure captured from the FDA adverse event reporting system (AERS). A detailed analysis of 50 cases showed 31 were due to infliximab and 19 etanercept. When other potential confounders (e.g., hepatitis, alcohol, MTX, hepatotoxic agents, GVHD) are excluded, 7 of 50 (14%) cases pointed to the TNF inhibitor as the lone offending agent.
An analysis by Gabril et al included 34 cases of TNF inhibitor-induced severe liver injury (3). The cohort included patients treated for psoriatic disorders (13 patients), inflammatory bowel disease (12), rheumatoid arthritis (6), and ankylosing spondylitis (3). Infliximab was the most common offender (26/34), but none were related to natalizumab, golimumab, or certolizumab use. The onset occurred in the first 3-6 months of therapy (range 2-104 wks) and two-thirds of patients were found to be ANA+ and anti-smooth muscle antibody (ASMA) positive. In many of the available liver biopsies showed a picture of autoimmune induced hepatocellular injury, but a mixed non-autoimmune pattern or predominant cholestasis pattern was also seen (3). A similar pattern was reported in a cohort of 11 patients from Iceland, who demonstrated drug-induced liver injury occurring mostly in infliximab treated patients, with half occurring after 4 infusions (4).
Drug-induced liver injury is a rare event, occurring in 20 per 10,000 patients per year (5). Evaluation of the patient should include hepatitis testing (especially hepatitis E) and look for common drug causes - Amoxicillin/clavulanate isoniazid, and nonsteroidal anti-inflammatory drugs are among the most common causes of DILI. Patients with a serum bilirubin >3.0 mg/dl have a 10% mortality risk.
There are no standard guidelines for managing severe liver injury with TNF inhibitors. Nevertheless, many sources suggest that TNF inhibitor treatment should be withdrawn and not repeated if the enzyme elevations are >5 times the upper limit of normal, or if the patient experiences jaundice. Elevated hepatic enzymes with TNF inhibitor use should prompt reassessment and careful review of other medications and comorbidities. Although it would be very unlikely for such elevations to be due to muscle inflammation, the adalimumab package insert states that 15% of children receiving ADA developed mild to moderate creatine kinase elevations. Those with marked elevations tend to respond well to discontinuation of the TNF inhibitor.
References







If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.