IL-23 Outduels IL-17 Inhibition in Psoriasis Save
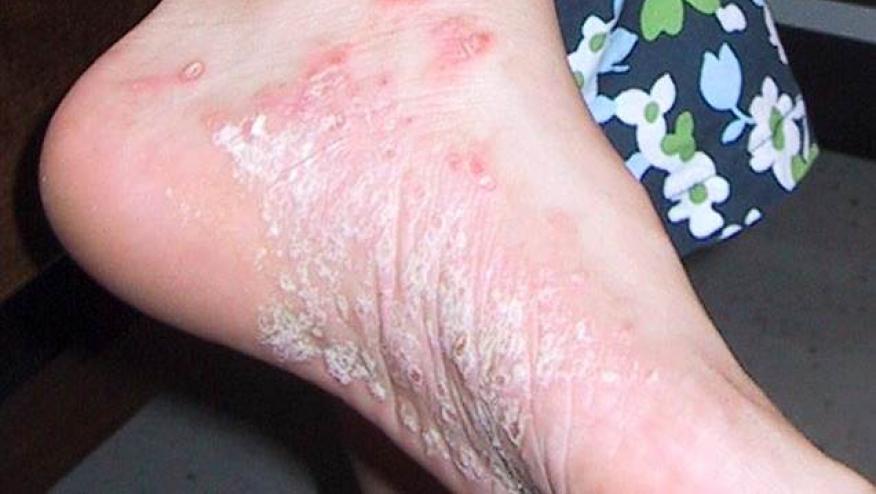
Johnson & Johnson has announced the preliminary results of its phase 3 ECLIPSE study; a head-to-head trial wherein guselkumab (Tremfya; an IL-23 inhibitor) was compared to secukinumab (Cosentyx; an IL-17 inhibitor) in adults with moderate to severe plaque psoriasis.
This study of 1,048 patients showed guselkumab to be significantly better than secukinumab. Guselkumab treated patients demonstrated PASI90 scores of 84.5% vs. 70% for those on secukinumab at week 48 (p<0.001).
The time to response, however, was similar between the two drugs.
According to Reuters, Tremfya, first approved in July 2017 as a plaque psoriasis treatment in the U.S., is also being tested as a treatment for psoriatic arthritis and Crohn’s disease. Cosentyx, which has been approved in the U.S. for plaque psoriasis, scalp psoriasis and psoriatic arthritis, is a key sales driver which raked in $2.1 billion for the drugmaker in 2017.






If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.