Stelara FDA Approved for Use in Adolescent Psoriasis Save
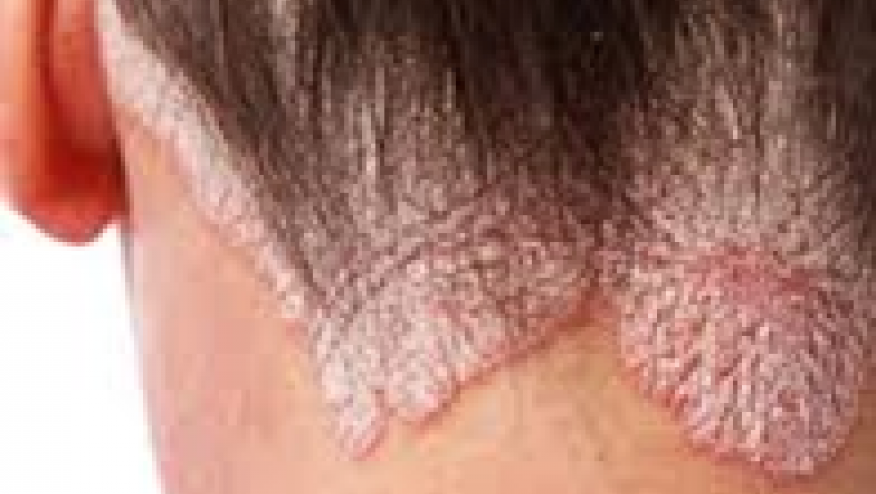
Johnson & Johnson has announced that they have received an expanded FDA approval for the use of ustekinumab (Stelara) in treating adolescent patients, aged 12 and over, with moderate to severe plaque psoriais.
Stelara is already approve for use in adults with psoriasis, psoriatic arthritis, Crohn's disease and now, in moderate to severe plaque psoriasis patients, who are candidates for phototherapy or systemic therapy. For those under 100 kg, the 45 mg dose is recommended (every 3 months)
Stelara is one of J&J’s most important pharmaceutical products, with 2017 sales expected to reach $3.7 billion and increasing to more than $5 billion by 2021, according to Thomson Reuters data.
Continue Reading
ADD THE FIRST COMMENT
Disclosures
The author has received compensation as an advisor or consultant on this subject






If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.