Targeting IL-17A: A Winner in PsA Save
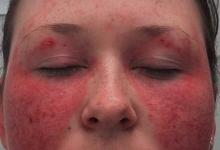
Psoriatic arthritis patients with inadequate response to tumor necrosis factor (TNF) inhibitors had improvements in their arthritis and psoriasis when treated with the interleukin (IL)-17A blocker ixekizumab (Taltz), according to a phase III study presented here at the annual conference of the British Society for Rheumatology.
Among 363 patients who were randomized to subcutaneous placebo or ixekizumab, 80 mg every 4 or 2 weeks following an initial baseline dose of 160 mg, significantly more of those those receiving ixekizumab showed ACR20 responses (20% improvements on American College of Rheumatology criteria) at week 24 compared with those given placebo, reported Helena Marzo-Ortega, MD, of the University of Leeds in England.
For those given 80 mg every 4 weeks, ACR20 responses were seen in 53.3%, while for those given 80 mg every 2 weeks, ACR20 responses were seen in 48%. In the placebo group, the response rate was only 19.5%.
In addition, ACR50 responses for the every 4 weeks, every 2 weeks, and placebo groups were 35.2%, 33.3%, and 5.1%, respectively, while ACR70 responses were seen in 22.1%, 12.2%, and 0% in the three groups. The study was sponsored by Eli Lilly and Company.
"Psoriatic arthritis is a chronic inflammatory condition which can be associated with extra-articular manifestations and metabolic morbidity," she explained. Approximately 30% to 40% of patients do not respond to anti-TNF therapy.
The high-affinity monoclonal antibody ixekizumab is approved for use in plaque psoriasis and has previously been shown to improve disease activity and physical function in patients with active PsA who had not received biologic treatment in a phase III study known as SPIRIT 1. The current study, SPIRIT 2, extended the treatment experience to patients who had not responded or were intolerant of TNF inhibition. A total of 56.2% had failed one TNF inhibitor, 35.3% had failed two, and 8.5% were TNF-inhibitor intolerant. More than one-third were on concomitant methotrexate.
The full 24 weeks of the trial was completed by 87% of participants; rescue therapy was available for nonresponders at week 16. Skin outcomes were assessed among patients who had involvement of at least 3% of their body surface area at baseline.
Patients' mean age was 52, and the sexes were evenly divided. The mean tender and swollen joint counts were 15 and 11, respectively.
Substantial improvements were already seen by week 2. "The response was very quick," she said.
A state of minimal disease activity was achieved at week 24 by 27.9% of the every 4 week group, by 23.6% of the every 2 week group, but by only 3.4% of the placebo group.
Significantly more patients who had 3% or more body surface area psoriasis at baseline had 75% improvements on the Psoriasis Area and Severity Index (PASI), with PASI-75 responses of 55.9% in the every 4 week group and 60.3% in the every 2 weeks group compared with 14.9% of the placebo group.
"One of the remarkable things was that nearly one-third achieved PASI-100s, and this is one of the first times we've seen this skin response," Marzo-Ortega said.
An itch numeric rating score of 0 was significantly more common in the ixekizumab groups, at 23.5% for both ixekizumab groups and none of the placebo patients. Significantly more achieved itch resolution or a dermatology life quality index score of 0 or 1 also was higher in the ixekizumab groups, at 32.4% of the every 4 week group and 25.6% of the every 2 week group compared with 3% of the placebo group.
For patient-reported outcomes, improvements also were seen in the active treatment groups on various indices including the Short Form 36 physical and mental component scales and the Work Productivity and Activity Impairment index.
More injection site reactions were seen in the active treatment groups, with most being mild. Other adverse events such as sinusitis and nasopharyngitis occurred with similar frequency in the three groups, at 68%, 73.2%, and 64.4%, respectively. There also were a couple of cases of esophageal candidiasis, she noted.
The study was sponsored by Eli Lilly and Company.





If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.