Time to Rethink Gout as a Chronic Disease Save
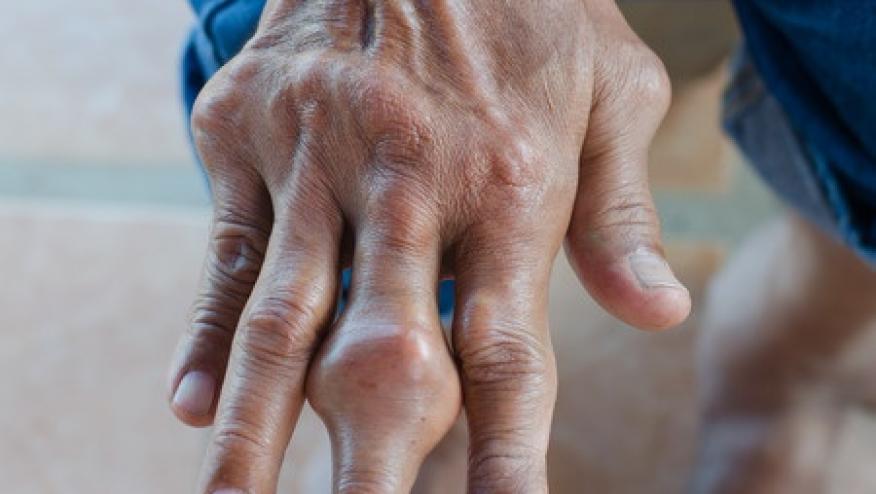
The current issue of JAMA has a perspective article on Gout’s bad rap as dietary disease rather than the complex, chronic inflammatory disorder that is ineffectively treated in many.
The author and experts interviewed believe that the pipeline of new drugs for gout will fuel the future of evidence-based care and more informed lifestyle instruction.
Gout affects about 4% of adults in the United States. The growing number of older adults, who are more likely to have gout, is expected to increase the need for gout care.
"The disease of kings" is clearly a misnomer as gluttony and excess seldom stand in the way of better care. Sadly, many with gout fail to recognize it as a form of arthritis, A 2016 online survey showed that 71% didn’t know gout and that half with gout were embarrassed by their condition.
Although diet is often implicated, evidence proving the benefits of strict avoidance are limited. While red meat, alcohol, sugar-sweetened soft drinks, and seafood have been implicated, the ACR guideline suggests that avoidance of purine-rich organ meats (liver), shellfish and sardines may only improve serum uric acid levels by10% to 18%.
Many experts have noted the limitations and pitfalls with found with a dietary approach to gout.
A real problem are the differing treatment approaches and understanding of gout by primary care physicians (PCPs) and rheumatologist, exemplified by their contrasting guidelines. The 2012 ACR focuses on a a treat-to-target approach with the goal of a uric acid levels below 6 mg/dL and the use of urate-lowering medications long-term with frequent monitoring. Yet the 2016 American College of Physicians guideline only has a treat-to-symptoms approach emphasizing the use of anti-inflammatory medications to control flares and reserving uric acid-lowering therapy for patients with frequent flares,
Discrepancies between the guidelines may be hampering patient care, and hopefully upcoming meetngs between the ACR and ACP may resolve these differences. The ACR’s gout guideline is scheduled for release in 2020.
Emerging evidence suggests that a chronic disease care model for gout, which simultaneously addresses uric acid levels, lifestyle, and dietary factors, may improve management and outcomes for patients with gout.







If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.