IL-17

1 year 10 months ago
#LB0005 #EULAR2022 Phase 3 RCT of Bimekizumab, dual IL17A and 17F inhibitor in bDMARDs-naive PsA showed significant improvement in ACR50 and PASI90 scores vs Placebo. No major safety signals. Skin response is excellent! @RheumNow https://t.co/yhgB8LGXh6

1 year 10 months ago
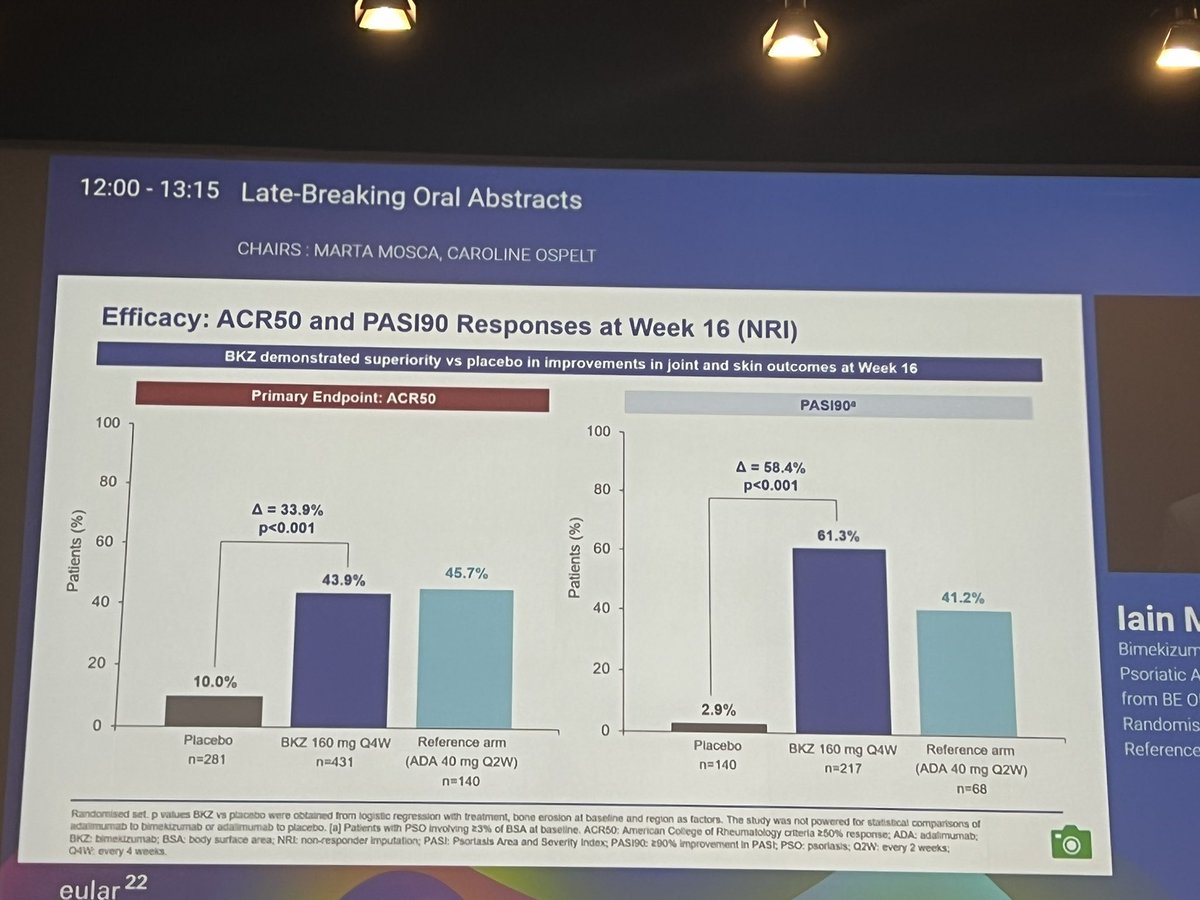
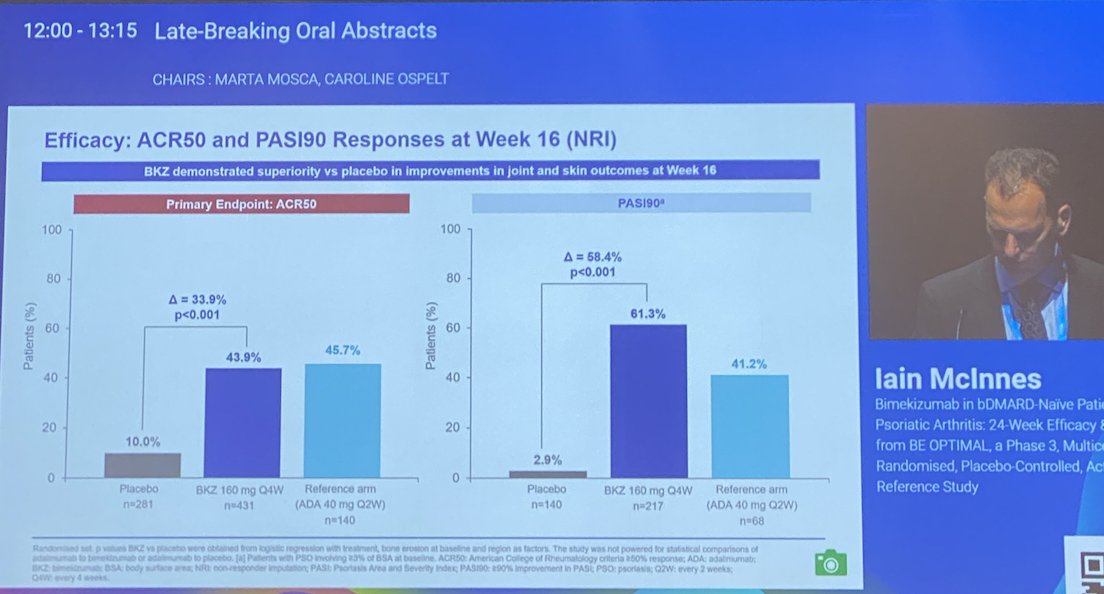
#EULAR2022 LB0001
Bimekizumab (IL-17F and IL-17A) in bionaive PsA
BE OPTIMAL phase 3 trial, 24 weeks
⭐️Met Primary endpt - ACR50
⭐️Met other ACR and PASI endpts
⭐️Safety: higher fungal infections
@RheumNow https://t.co/x8u6B93FNl

1 year 10 months ago
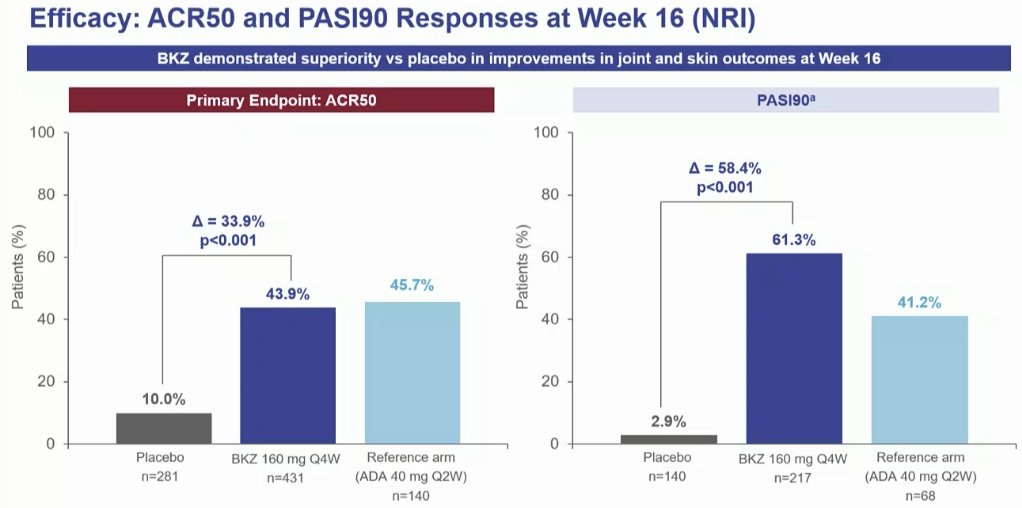
BE OPTIMAL Phase 3 RCT
Bimekizumab dual IL17A & F inhibitor in bionaive PsA
⭐️ACR50 wk 16 43.9% vs 10% PBO
⭐️PASI 90 wk 16 61% vs. 3% PBO
⭐️separation from wk 4
Safety: fungal injections BKZ > ADA
@RheumNow
LB0001 #EULAR2022 https://t.co/7ZwV1XDSNb

1 year 10 months ago
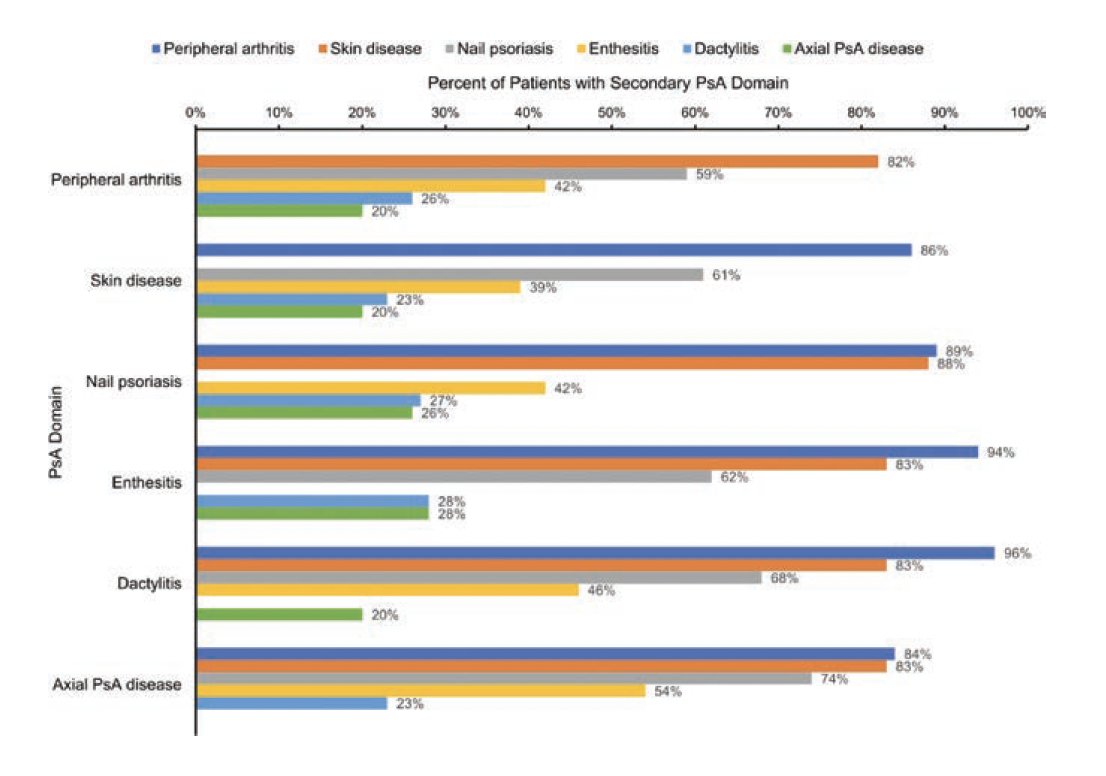
CorEvitas PsA Registry (1000+ pts)
Analysis of biologics prescription according to disease domains
No real surprise to see that IL-17i were prescribed more frequently in pts w/ PsO BSA>10% at BL
Overall first line TNFi 40% > IL-17i 14%
POS0309 @RheumNow #EULAR2022 https://t.co/dkLxl8d6ug

1 year 10 months ago
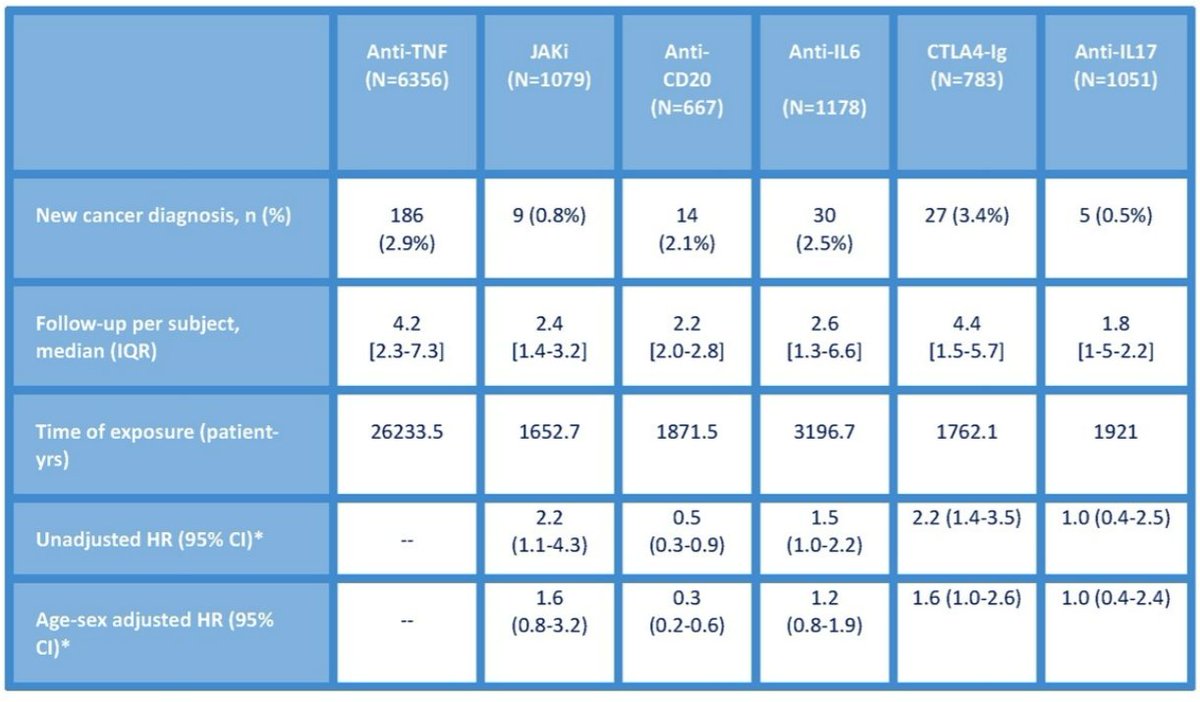
Castrejon et al. BIOBADASER registry study. Cancer risk with various bDMARDs. Overall I don't think there is a difference here. Lowest in IL17i and highest in abatacept but older and comorbidity confounders @RheumNow #EULAR2022 #POS1439 https://t.co/gfv78ouOTJ
By now, those of us attending the meeting know how to find a free coffee or sprite, have found comfortable meeting nooks and know our way around (lots of sneaker mileage)! And congrats to…

1 year 10 months ago
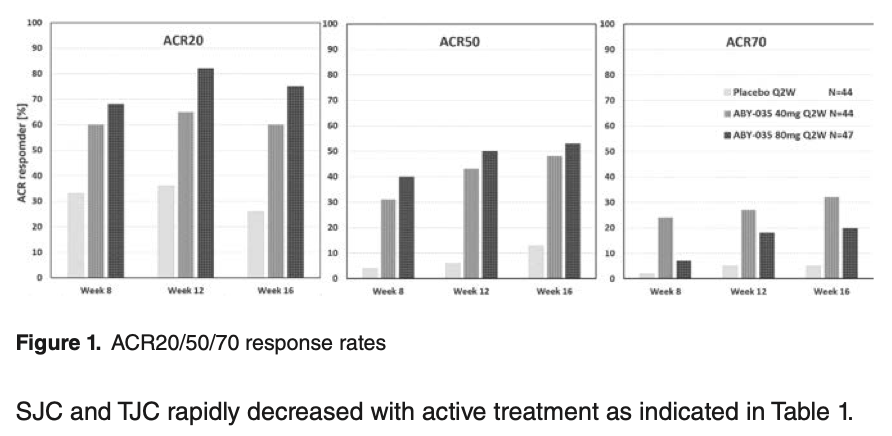
Izokibep - potent and small molecule IL-17A inhibitor Phase 2 study in PsA patients
Fast onset, met ACR50 primary endpoint
AE included site reaction and candida infection
@RheumNow #EULAR2022 ABST#OP0258 https://t.co/vZ4xmnhyhl

1 year 10 months ago
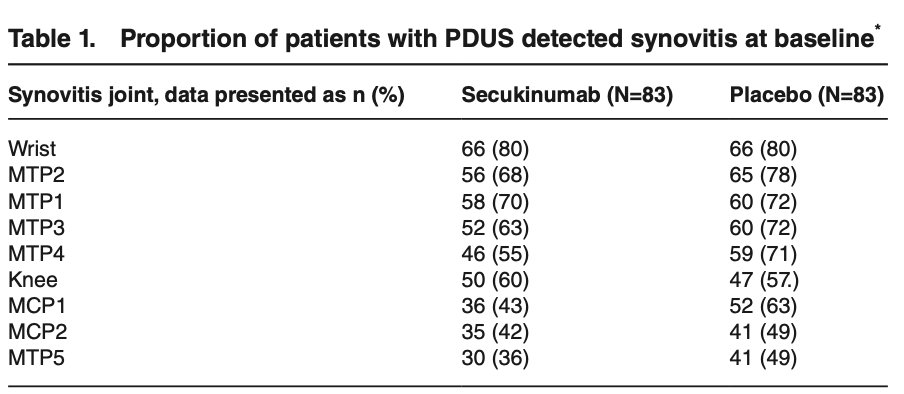
52 wk trial using MSK Ultrasound study on PsA receiving secukinumab showed an early and continued improvement in GLOESS scores.
Synovial hypertrophy most responsive.
Hands and feet, wrist and knees most affected and responsive.
@RheumNow #EULAR2022 ABST#OP0260 https://t.co/jeQezIWLxa

1 year 10 months ago
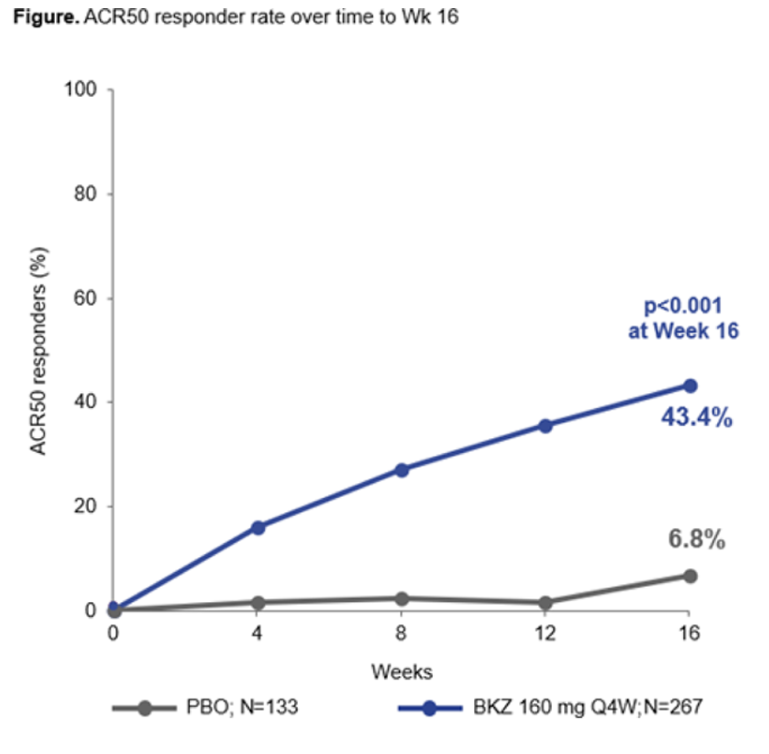
Merola et al. BE COMPLETE trial of bimekizumab (dual 17A/17Fi) in TNF-IR PsA. Week 16 ACR50: 43.4% BKZ vs 6.8% PBO. Rapid response with separation from week 4. @RheumNow #EULAR2022 OP0255 https://t.co/ZaxAeePP6L https://t.co/D5mDdkno3c

1 year 10 months ago
Behrens et al. Izokibep (small molecular size IL17Ai, suggested to abrogate issues with tissue distribution of monoclonal abs) in PsA. ACR50 52% in 80mg, 48% in 40mg, 13% in PBO. @RheumNow #EULAR2022 https://t.co/FUS50ax79C https://t.co/e1n1RbNw1b













 Poster Hall
Poster Hall