Celebrex Goes Generic Save
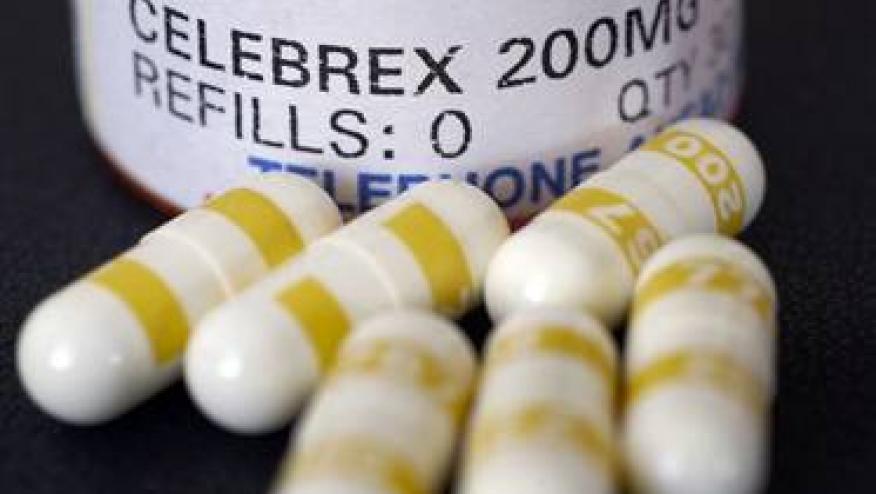
The generic drug manufactuer Actavis joins Teva and Mylan with their lauch of celecoxib (Celebrex)in 50 mg, 100 mg, 200 mg and 400 mg capsules. In 2013 celecoxib was the 21st leading prescribed drug in the U.S. with $2.4 billion in sales as of mid 2014. This article reviews the potential financial impact of this change on Actavis and Pfizer.









