Recent News
 Read Article
Read ArticleDr. Jack Cush reviews this past week’s news and journal articles from RheumNow.com.

Links:
Dr. John Cush @RheumNow ( View Tweet)

Links:
Dr. John Cush @RheumNow ( View Tweet)

Links:
Dr. John Cush @RheumNow ( View Tweet)

Links:
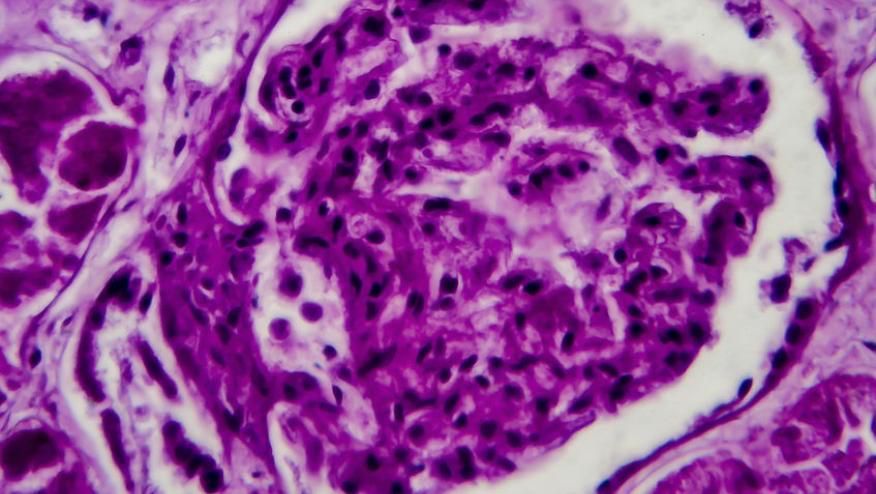
Predicting Infection in Lupus Nephritis
Machine learning (ML) models were applied to 3 cohorts of lupus nephritis (LN) patients and established algorithms to predict co-infection in LN.
This study analyzed 111 non-infected LN patients, 72 infected LN patients, and 206 healthy controls (HCs), looking at patient demographics, infection outcomes, medication, and laboratory indexes (including cell counts and lymphocyte subsets).
Read Article
Links:
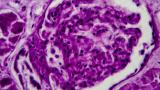
Predicting CKD Outcomes in Lupus Nephritis
An observational cohort study suggests that proteinuria levels in lupus nephritis (LN) patients did not predict their histologic class. A multicentre observational study of patients with biopsy-proven LN; either proliferative (PLN) or membranous (MLN) lupus nephritis were compared regarding clinical and laboratory presentation and long-term outcomes. The primary outcome was progression to chronic kidney disease (CKD).
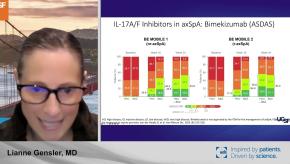
Read ArticleICYMI: Five Takeaways in PsA/SpA at RNL 2024
Continuing with the theme of clinical pearls, the PsA and SpA speakers also provided a wealth of information. Here are my five key takeaways and clinical pearls.
Read Article










 Poster Hall
Poster Hall