Anti-IL-5 Success in Eosinophilic Granulomatosis with Polyangiitis Save
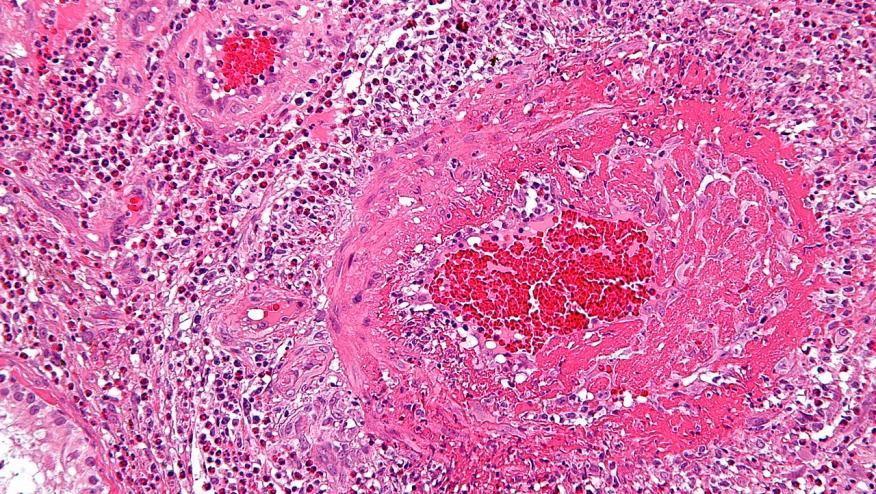
The NEJM reports success when using mepolizumab (anti-IL-5 monoclonal antibody) in a 52 week study of patients with eosinophilic granulomatosis with polyangiitis (EGPA).
EGPA, previously known as Churg-Strauss vasculitis is an eosinophilic vasculitis that has often been managed as other systemic necrotizing vasculitis. Given the eosinophilic component, Weschler and coworkers studied the efficacy and safety of mepolizumab, an anti–interleukin-5 monoclonal antibody, in EGPA.
The NEJM reports on a 136 patients study with relapsing or refractory EGPA patients on stable doses of steroids were randomized to either 300 mg of mepolizumab or placebo, administered subcutaneously every 4 weeks, for 52 weeks. The two primary end points were the accrued weeks of remission over a 52-week period, and the remission rates at both week 36 and week 48.
The mepolizumab were 6 times more likely to have more accrued more weeks of remission than placebo (28% vs. 3%).
The anti-IL-5 mAb also had a higher percentage of remissions at both week 36 and week 48 (32% vs. 3%). Non-remission was seen in 47% on mepolizumab compared to 81% on placebo.
The annualized relapse rate 50% lower in the mepolizumab group, compared to the placebo group and there was less steroid use in the active treatment group, with 18% of the mepolizumab patients being able to discontinue prednisone completely..
Although only half the participants treated with mepolizumab had protocol-defined remission, this novel biologic intervention represents a new and significant advance in the treatment of EGPA.
An accompanying NEJM editorial by Drs.Djukanovic and O'Byrnes (http://buff.ly/2qW3qsJ) commented "Although the trial was powered to evaluate relapses on the basis of a worsening condition in any of three categories (asthma, vasculitis, and sinonasal disease alone or in combination), the benefit of treatment was slightly greater with regard to relapses defined according to exacerbating asthma-based or sinonasal-based symptoms. This finding suggests that the vasculitic component of eosinophilic granulomatosis with polyangiitis may respond less well to mepolizumab than do other components of the disease." They comment that additional trials with this agent are needed and that further study of its possible synergism azathioprine, cyclophosphamide or other immunosuppressants would be welcomed..





If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.