CDC: Outbreak of Arthrocentesis Related Septic Arthritis Cases in New Jersey Save
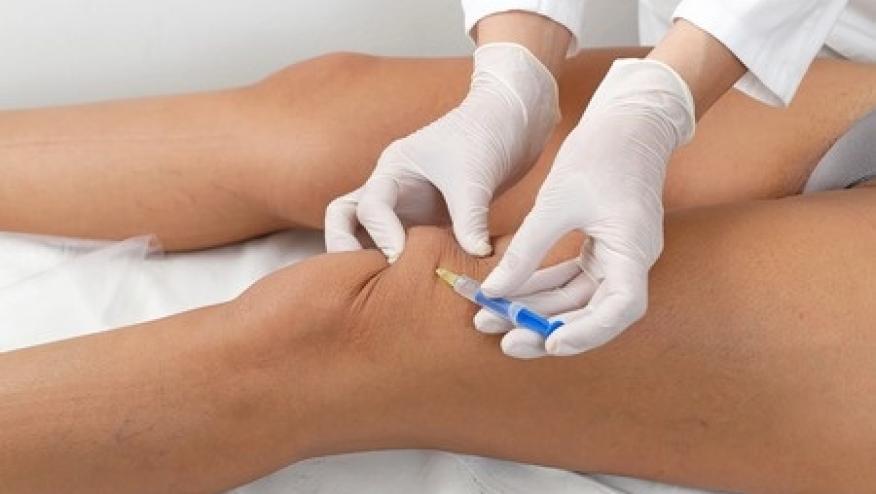
It is estimated that the risk of septic arthritis following joint aspiration or injection is roughly 1:50,000. While many injectable medications come with antimicrobial preservatives, the use of single-use medications, including pharmacy bulk packaged (PBP) products, may not include such preservatives and can become contaminated if handled inappropriately.
In March 2017, an outbreak of 41 cases of septic arthritis associated with intra-articular injections administered at an single outpatient practice occurred in New Jersey. A public health investigation identified multiple breaches of recommended infection prevention practices during the preparation and administration of PBP products. The intraarticular injections were done using fluoroscopic visualization. The CDC has reported the results of its investigation.
On March 6, 2017 the Monmouth County Regional Health Commission was notified by the NJ Dept. of Health that three patients were hospitalized for septic arthritis after receiving intra-articular injections for osteoarthritis of the knee. The practice involved two staff physicians and two medical assistants.
From this clinic and one provider, there were 250 patient visits for knee intra-articular injections between March 1 and March 6, that lead to 41 confirmed cases (16%) of septic arthritis. Symptoms were identifed from zero to 65 days after injection, with 92% having symptoms within 48 hours of the procedure. Thirty (73%) of the 41 patients required surgery.
Synovial fluid cultures were positive for in 37% of patients, with the following bacteria: Streptococcus mitis-oralis (10 patients), Abiotrophia defectiva (two), Staphylococcus aureus (two), Actinomyces odontolyticus (one), alpha-hemolytic Streptococcus (one), Eikenella corrodens (one), Haemophilus parainfluenzae (one), Neisseria oralis (one), Streptococcus gordonii (one), Streptococcus intermedius-milleri (one), Streptococcus sanguinis (one), and Veillonella (one); five patients had polymicrobial infections. Many of the cultured organisms are commonly found in oral flora. Staphylococcus aureus was isolated from the blood of two patients.
Numerous infection prevention errors were identified including:
- staff members did not have access to a handwashing sink
- alcohol-based hand rub was not available in medication preparation or treatment areas
- staff members were operating under the mistaken belief that PBP products could be used as multiple-dose containers outside of pharmacy conditions (e.g., use of a laminar flow hood, appropriate garbing, staff training, and environmental monitoring)
- staff accessed the same 50 mL PBP container up to 50 times to prepare syringes for multiple patients (with the septum of the container cleaned with alcohol only before the initial draw)
- staff members prepared injections in a separate room, away from the patient treatment area without the use of sterile conditions
- injectable medications were drawn into syringes by medical assistants up to 4 days in advance of procedures
- Injections were initiated using a needle and syringe filled with local anesthetic. After injecting the anesthetic, the physician removed the syringe, leaving the needle within the intra-articular space. A second syringe containing contrast material from the PBP container was then attached to the needle hub and used to facilitate fluoroscopic needle placement. This was followed by replacement with a third syringe containing a glucocorticoid or hyaluronic acid–based product
- The physician did not wear a face mask during joint injection procedures and used nonsterile gloves to manipulate the needle hub during procedures
The practice was advised to immediately stop batch preparation of syringes and use of PBP products for multiple patients and to hire an infection preventionist to assess staff competency and ensure that hand hygiene, standard precautions, and safe injection practices were followed. No additional cases occurred after these measures were implemented.
Once infection prevention recommendations were implemented in the practice, no additional septic arthritis cases were reported.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.