CDC Top 15 Most Common Opioid Overdose Drugs Save
The Dec. 12 issue of the National Vital Statistics Reports from the U.S. Centers for Disease Control and Prevention reports that the most commonly abused drugs causing drug overdose deaths (between 2011-2016) include fentanyl, heroin, oxycodone, and cocaine.
From 1999 through 2016, the age-adjusted rate of drug overdose deaths in the United States more than tripled from 6.1 per 100,000 to 19.8 per 100,000 according to the report.
The top 15 drugs were identified based on the number of drug overdose deaths per referent drug category. While the ranking changed from year to year, the top 10 drugs involved in overdose deaths remained consistent throughout the 6-year period. The top 10 drugs belonged to three drug classes:
- Opioids: fentanyl, heroin, hydrocodone, methadone, morphine, and oxycodone
- Benzodiazepines: alprazolam and diazepam
- Stimulants: cocaine and methamphetamine
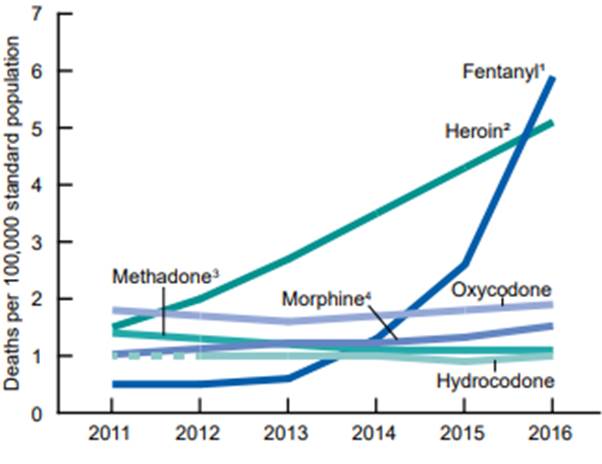
In 2011, oxycodone ranked first, while heroin and fentanyl were ranked first during 2012 to 2015 and in 2016, respectively.
Cocaine consistently ranked second or third during the study period. The age-adjusted rate of drug overdose deaths involving heroin more than tripled from 2011 through 2016, as did the rate of drug overdose deaths involving methamphetamine.
Each year from 2013 to 2016, the rate of drug overdose deaths involving fentanyl and fentanyl analogs doubled (0.6, 1.3, 2.6, and 5.9 per 100,000 in 2013, 2014, 2015, and 2016, respectively).
This report shows that the most frequent drugs mentioned varied over time and by intent of death (i.e., unintentional drug overdose, suicide by drug overdose, and overdose death of undetermined intent). Results from the literal text analysis also confirm that many drug overdose deaths involve multiple drugs.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.