Cochrane Review: Calcium Channel Blocker Efficacy in Raynaud's Save
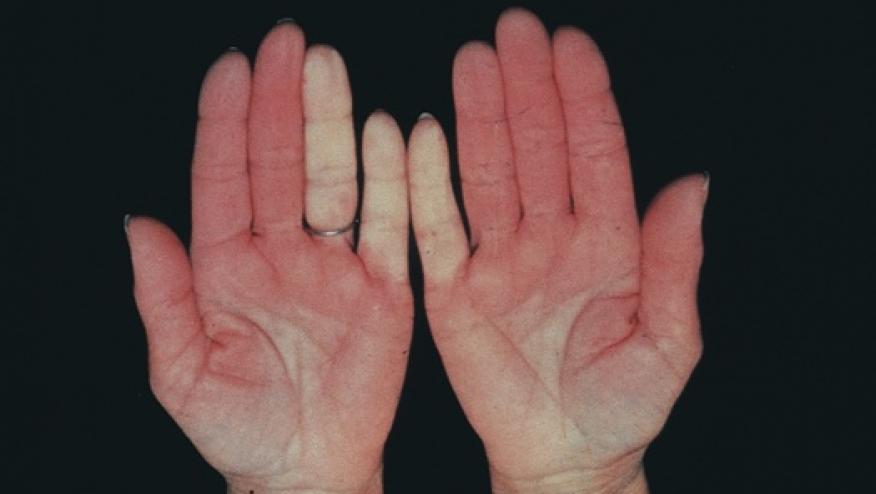
The Cochrane Database has published its review of calcium channel blockers (CCB) in Raynaud's phenomenon, showing CCBs may be useful in reducing the frequency, duration, severity of attacks, pain and disability associated with Raynaud's phenomenon, especially with primary Raynaud's.
In their review they distinguished primary Raynaud's phenomenon (not associated with underlying disease) from secondary Raynaud's phenomenon (associated with connective tissue disorders).
They analyzed data from 1946 to 2017, specifically looking for randomized controlled trials (RCTs) comparing calcium channel blockers versus placebo.
They identified 38 RCTs (33 cross-over RCTs) with an average duration of 7.4 weeks and 982 participants.
Nine of the identified trials studied patients with primary Raynaud's phenomenon (N = 365), five studied patients with secondary Raynaud's phenomenon (N = 63), and the rest examined a mixture of patients with primary and secondary Raynaud's phenomenon (N = 554).
Looking at both primary and secondary Raynaud's phenomenon, evidence (moderate quality) from 23 trials with 528 participants shows CCBs were superior to placebo in reducing the frequency of attacks, severity of attacks, and Raynaud's pain (low-quality evidence).
The most common side effects were headache, dizziness, nausea, palpitations, and ankle edema. However, in all trials, no serious adverse events (death or hospitalization) were reported.
Randomized controlled trials with evidence of low to moderate quality showed that CCBs (especially the dihydropyridine class) may be useful in reducing the frequency, duration, severity of attacks, pain and disability associated with Raynaud's phenomenon. Higher doses may be more effective than lower doses and these CCBs may be more effective in primary RP. Although there were more withdrawals due to adverse events in the treatment groups, no serious adverse events were reported.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.