Defining Refractory Rheumatoid Arthritis Save
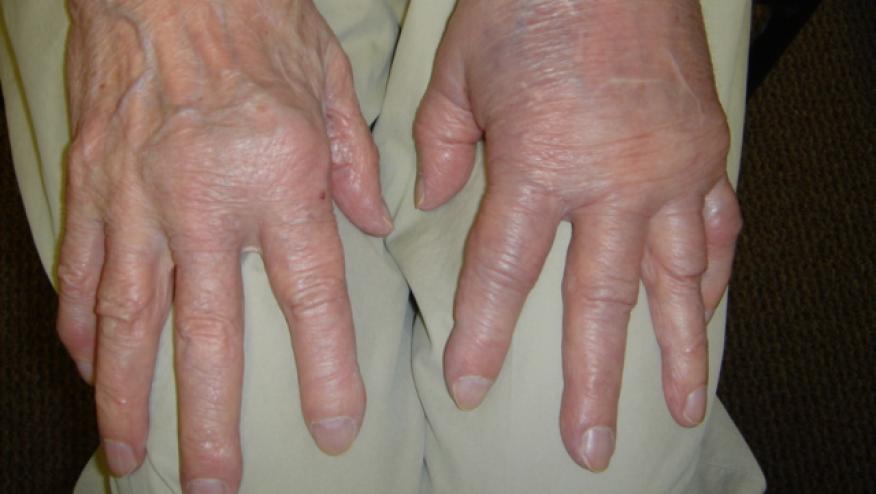
Dr. Maya Buch from Leeds has a thoughtful Viewpoint article in Annals of Rheumatic Disease on how to consider and manage the refractory or difficult rheumatoid arthritis (RA) patient.
Despite advances in our understanding the pathogenesis of RA and the advent of novel biologic and small molecule disease-modifying antirheumatic drugs, we still have a regretful proportion of patients who are refractory to our best therapies.
While the topic is often discussed and usually lapses into the potential hope created with a newly approved RA drug, there has been little discussion about what defines difficult or refractory RA - phenotypically or otherwise.
The results of randomised controlled trials show that approximately 20% progress onto a third bDMARD with a more modest proportion failing additional bDMARDs.
Dr. Buch suggests research and discussion and proposes key features that define refractory RA:
- resistance to multiple therapeutic drugs with different structures and mechanisms of action
- failure of at least one anticytokine (TNF and/or IL-6 directed) and one cell-targeted (B cell depletion and/or T cell costimulation blockade) bDMARD (the introduction of JAK inhibitors may need to be incorporated into this equation)
- intrinsic refractory: persistent inflammation, no anti-drug antibodies (ADA) (with/without secondary damage)
- pharmacokinetic refractory: persistent inflammation with ADA
- false refractory: absence of inflammation; other (biomechanical±degenerative) drivers.
Based on these, refractory RA patients may be considered to be: a) intrinsically refractory; b) PK refractory (ADA+); or c) false positive refractory (no inflammatoin, no ADA), and therefore have other issues (e.g., mechanical etc.) underlying the patients continued nonresponse.






If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.