Disparities in Lupus Survival Save
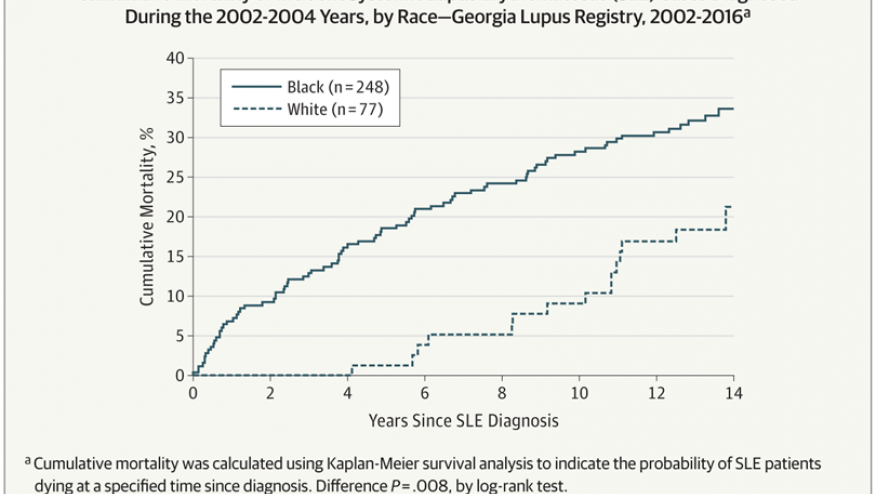
MMWR has published the outcomes from the Georgia Lupus Registry between 2002 and 2016, finding that black women were not only more likely to die from lupus than white lupus patients; but they died on average 13 years earlier (mean age 51.8 and 52.3 years, respectively) than whites (mean age 64.4 and 65.0 years, respectively).
Black women with lupus were 3.34 times more likely to die than black women in the general population, while white women with lupus were 2.43 times more likely to die than white women in the general population. None of the white women with lupus died within 5 years of diagnosis, while mortality was elevated for black women from the date of diagnosis on.
Systemic lupus erythematosus (SLE) is a chronic, systemic autoimmune disease that disproportionately affects women and minorities. Blacks with SLE also have more severe disease and develop it at an earlier age.
During 2002–2004, a total of 336 incident SLE cases were identified; these SLE patients were demographically similar to the patients in 1,353 cases with prevalent SLE in 2002 (87%–90% female, 74%–76% black, and 23% white) but were older at SLE diagnosis (mean age 40.6 years) than were patients with prevalent SLE (34.6 years). Among patients with prevalent and incident SLE, 401 and 97 deaths, respectively, occurred through 2016. Standardized mortality ratios using 2002–2016 data were 2.3–3.3 times higher for persons with prevalent SLE relative to expected deaths in the general population (Table). Black females with prevalent SLE were three times more likely to die than were black females in the general population (standardized mortality ratio = 3.38). Cumulative mortality was significantly higher among blacks than among whites for both incident (Figure 1) and prevalent (Figure 2) SLE; death occurred at a younger age among blacks with incident SLE cases (mean age = 51.8 ± 17.5 years) and prevalent SLE cases (mean 52.3 ± 15.9 years) than it did among whites (64.4 ± 18.9 years and 65.0 ± 16.3 years, respectively) (p0.001). Mortality among whites was markedly lower in the years immediately following diagnosis compared with mortality among blacks; among incident cases, no deaths were observed among whites until 5 years after SLE diagnosis, whereas mortality among blacks was persistently higher from the time of diagnosis. In addition, whites with SLE had the same cumulative mortality proportion (9%) in 10 years as that observed in blacks in 2 years (Figure 1). There were no significant differences by sex.
Despite increasing awareness of SLE and advancements in treatment, mortality among persons with SLE remains high, with the highest standardized mortality ratio among black females. The effect of this racial disparity in mortality is further underscored by the fact that the prevalence of SLE in blacks is three times that in whites.
The CDC is funding longitudinal studies through the Georgia Lupus Registry, California Lupus Surveillance Project, and Michigan Lupus Epidemiology and Surveillance Program and also participating in the funding efforts of the Lupus Foundation of America and the American College of Rheumatology.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.