Effective Interferon-Free Regimen for HCV-Cryoglobulinemic Vasculitis Save
Rheumatologists have been challenged to treat hepatitis C virus (HCV)-induced rheumatic disease since discovery of the virus in 1989. For years, standard treatment was interferon (IFN)-based and associated with significant toxicity and a low likelihood of virologic cure (5% for IFN monotherapy to 50% with PEGylated IFN plus ribavirin).
Over the years, a series of steps have brought new and improved treatment options of HCV. After the introduction of several poorly tolerated direct acting agents, a number of new, oral, well-tolerated, IFN-free combination treatments have revolutionized the treatment of HCV with cure rates approaching 100% in just 3 months or less.
The question has been, what will these new dramatic therapies mean for the treatment of extrahepatic manifestations of HCV?
A French study published earlier this year in Annals of Rheumatic Disease evaluated the safety and efficacy of sofosbuvir 400 mg/day, a nucleotide polymerase inhibitor, plus ribavirin 200-1400 mg/day for 24 weeks to treat HCV-cryoglobulinemic vasculitis (CV), a rare yet serious complication of chronic HCV infection.
They enrolled 24 patients with active HCV-CV, excluding patients with HBV, HIV or decompensated cirrhosis. The primary endpoint was a complete clinical response of CV at week 24. Secondary endpoints included sustained virological response (SVR) at week 12, course of cryoglobulinemia, C4, ALT, the Short Form Health Questionnaire and side effects through 36 weeks of follow up.
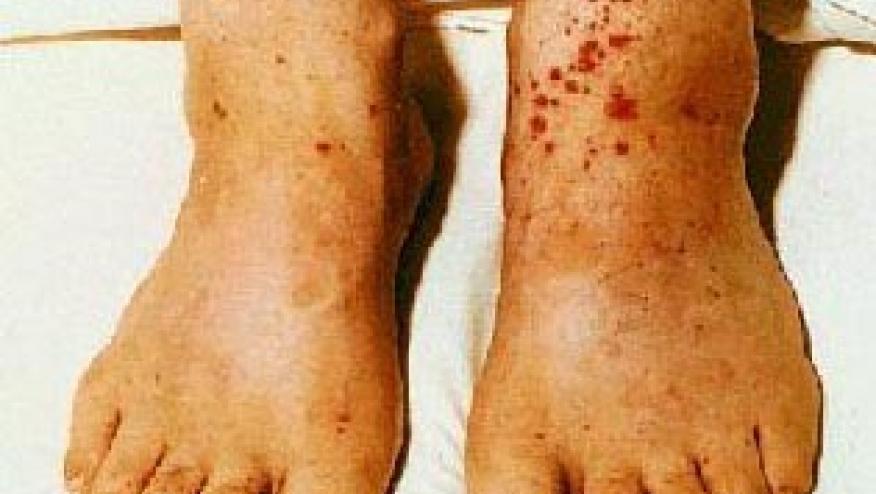
The main clinical features of CV were purpura, peripheral neuropathy, arthralgia, glomerulonephritis and skin ulcers. 80% of patients had type II mixed cryoglobulins. Seven patients had been treated with immunosuppression for kidney disease and/or neuropathy with combinations of rituximab, IVIG and glucocorticoids. Eleven patients were naïve to antiviral treatment for HCV and 13 were previous non-responders to presumably IFN-based therapies. They found that at 12 weeks 74% experienced SVR, and 91.7% did at week 24.
A complete clinical response was seen in 21 (87.5%) of patients at week 24. Purpura, skin ulcers and arthralgia resolved in all cases, and renal disease resolved in 4 out of 5. Cryoglobulin levels disappeared in 46.1% of cases. 58% experienced any AE with the most common being fatigue, insomnia and anemia. There were 2 serious AEs.
Overall, their results are highly encouraging and contrast with previous treatment options such as first generation protease inhibitors with peg interferon and ribavirin, where SVR12 was only ~50%.
At the time this study was designed, sofosbuvir with ribavirin was the best available interferon-free regimen. Since then, many new agents have been approved but have yet to be tested in HCV-CV. For example, sofosbuvir/velpatsavir, the most recently approved combination, is a fixed-dose combo pill for genotypes 1-6, the only pan-genotypic regimen in existence, and boasts a SVR12 nearing 100% (Citation http://buff.ly/2dXsBDS). While untested in HCV-CV we would expect this combination to be even more effective and less toxic in this setting. Of course HCV-CV patients are complex and may have significant renal disease which will affect therapy options for some.
So, what is the take away here? Direct-acting agents are effective, we no longer need IFN, and this is only the tip of the iceberg. When you find patients with HCV and cryoglobulinemia, work with the hepatologist and you may cure them of HCV.







If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.