Enbrel Approved for Pediatric Chronic Plaque Psoriasis Save
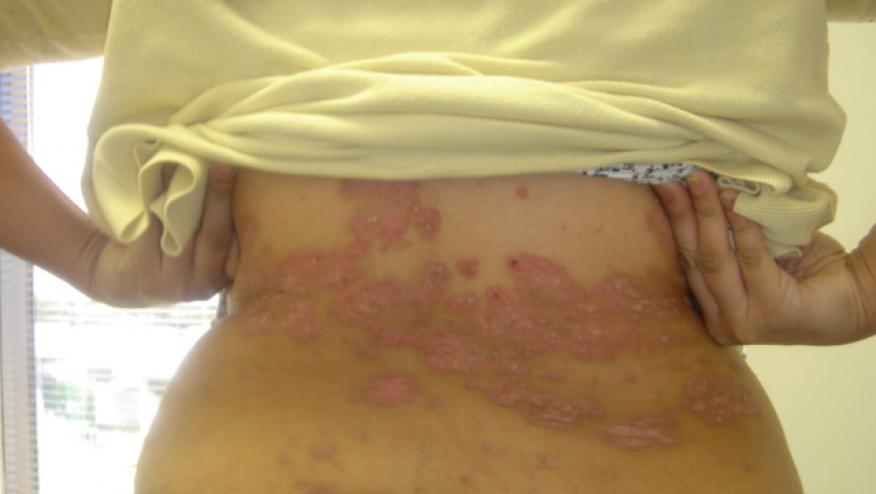
Etanercept (Enbrel) received FDA approval Friday for use in children with moderate to severe, chronic plaque psoriasis. Previously approved for adults with plaque psoriasis the new indication will be for chronic plaque psoriasis for those over age 4 years. Etanercept is now the first systemic therapy approved to treat pediatric psoriasis patients (ages 4-17).
Etanercept is already approved for adult rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and polyarticular juvenile idiopathic arthritis (JIA) aged 2 years or above.
The approval is based on results from a Phase 3 one-year study and its five-year open- label extension study to evaluate the safety and efficacy of etanercept in pediatric patients, ages 4 to 17, with chronic moderate-to-severe plaque psoriasis.
In addition to demonstrating significant efficacy, the adverse events were similar to those seen in previous studies in adults with moderate-to-severe plaque psoriasis. "
"The need for an effective treatment for chronic moderate-to-severe pediatric psoriasis patients is high, and safety is always a concern when it comes to treating children. with moderate-to-severe plaque psoriasis,” said Sean E. Harper, M.D., executive vice president of Research and Development at Amgen.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.