Enthesitis in PsA: A New Focus of Treatment? Save
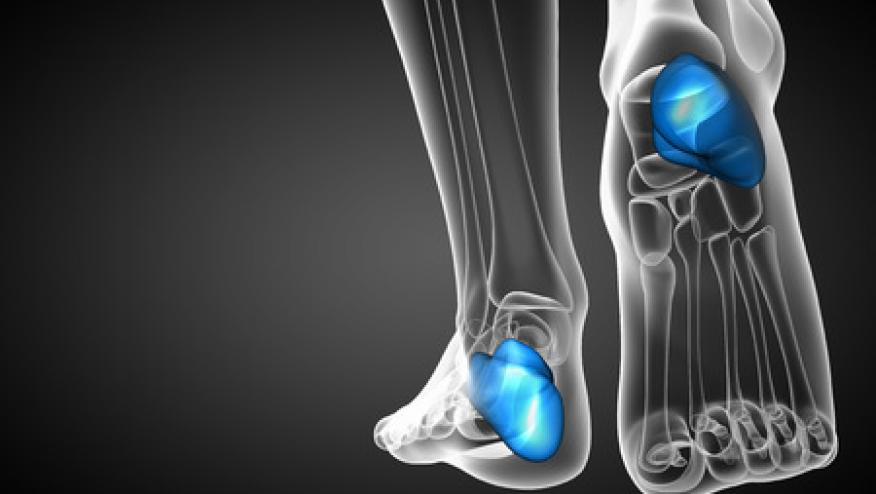
Patients with psoriatic arthritis (PsA) whose disease manifestations include enthesitis showed significantly greater responses to interleukin (IL)-23 inhibition than to blocking tumor necrosis factor (TNF), suggesting the possibility that treatment decisions in the future might be guided by specific cytokine targeting.
In a small, open-label study, clearance of enthesitis as defined by a score of zero on the Spondyloarthritis Research Consortium of Canada (SPARCC) index was achieved by 73.9% of patients receiving the IL-12/23 blocker ustekinumab (Stelara) by week 24 compared with 41.7% of patients receiving TNF inhibitors (P=0.018), according to Georg Schett, MD, and colleagues from University Clinic of Erlangen-Nuremberg in Germany.
Yet the responses for the arthritis component of disease was similar in the two groups, with clearance of both joint swelling and pain seen in 41% of the ustekinumab and 34% of the anti-TNF group, the researchers reported in Seminars in Arthritis & Rheumatism.
Clinically active enthesitis -- inflammation surrounding the tendon insertion sites -- has been reported in up to 50% of patients with PsA, yet almost all clinical trials of PsA treatment have focused exclusively on polyarticular arthritis, using primary endpoints such as the American College of Rheumatology 20% improvement criteria (ACR20). Patients in those studies were not required to have enthesitis, and when it was present, it was included only in subanalyses.
"New concepts suggest that enthesitis is based on a distinct molecular pathology. For several reasons, these data suggest a central role of the pro-inflammatory cytokine IL-23 in the development of enthesitis," Schett and colleagues explained.
Therefore, to examine the effects of inhibiting that cytokine, the researchers conducted a prospective, randomized study that included 47 patients with active enthesitis and failure of treatment with methotrexate at doses up to 25 mg/week for 3 months.
Participants were randomized to receive standard doses of subcutaneous ustekinumab or a TNF inhibitor, with the choice of TNF inhibitor according to patient preference as to route and frequency of dosing. A total of 10 patients received adalimumab (Humira), six were given certolizumab pegol (Cimzia), five received etanercept (Enbrel), and three were treated with infliximab (Remicade).
Patient characteristics were largely comparable in the ustekinumab and anti-TNF groups, with a mean age of 60 and disease duration of 2 to 3 years, although more men than women were included. Baseline SPARCC score was 4, and Disease Activity Score (DAS) 28 was 4.2.
Enthesitis was also measured with two other instruments, the Maastricht Ankylosing Spondylitis Enthesitis Score and the Leeds Enthesitis Index. On those measures, clearance of enthesitis by week 24 was seen in 82% and 78%, respectively, of patients in the ustekinumab group compared with 50% for both among those in the anti-TNF group.
Complete remission of skin disease, defined as a score of 100 on the Psoriasis Area and Severity Index (PASI100), was seen in 59% of the ustekinumab group compared with 29% of the TNF inhibitor group (P=0.039), while PASI90 responses, or near resolution, was observed in 86% and 29%, respectively (P=0.0001).
Complete resolution of enthesitis, arthritis, and psoriasis was reported in 18% of the ustekinumab group and 8% of the anti-TNF group.
The researchers also looked at factors that might be predictive of clearance of enthesitis, including age, sex, duration of disease, severity of skin and joint involvement, and treatment with ustekinumab or a TNF inhibitor, and only treatment of ustekinumab showed a significant association (OR 0.025, P=0.006).
"In summary, these data generate the hypothesis that there may be pathogenic differences with stratified therapeutic implications between synovitis and enthesitis in PsA, requiring further larger trials to validate this concept. We show that enthesitis-driven PsA patients are highly susceptible to blockade of IL-12/23, suggesting the feasibility for a more stratified treatment approach in PsA patients in the future," they concluded.
Limitations of the study, the investigators said, included the small number of patients and the lack of blinding.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.