Factors That Contribute to Uveitis in SpA Save
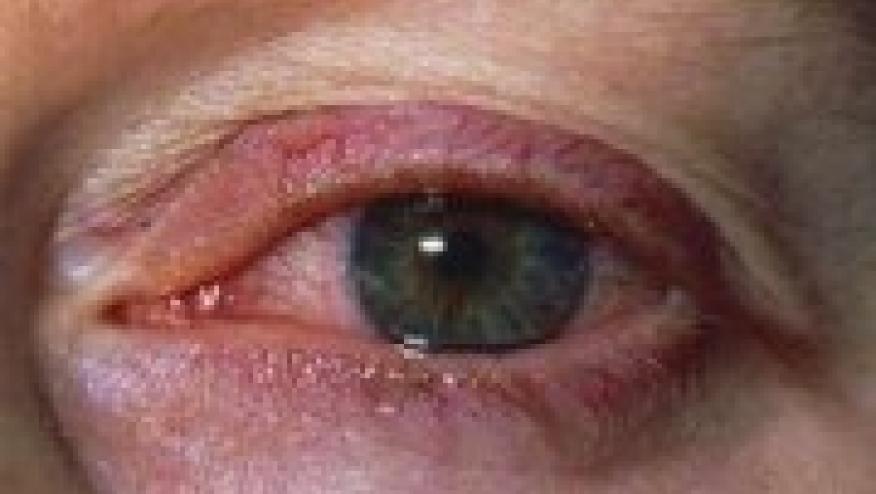
We know that uveitis is an extra-articular manifestation of SpA but what remains unclear is if there are any factors that may play a role in the development of uveitis. One group sought to understand these issues.
Dr. Kasifoglu et al sought to understand possible risk factors by looking at age, gender, duration of illness, BMI, educational status, smoking habits, SpA clinical findings and co-morbid disease, HLA B27 status, acute phase responses, VAS pain, VAS global, VAS fatigue, BASDAI and BASFI scores, swollen and tender joint counts and XR findings retrospectively.
The study looked at a total of 2359 SpA patients (2096 with axSPA, 1249 with AS, 100 with nr-axSpA, 179 with PsA, 50 with peripheral SPA and 51 with enteropathic arthritis.) Overall, 269 (11.4%) patients had experienced one or more episodes of uveitis. The median age of uveitis was 36 year old, with the start of SpA symptoms 6.8 years prior to uveitis. The median number of “attacks” was 2 (1-4) and were typically unilateral (78.9%.) 19/184 (10.3%) patients had permanent damage to the eye. These patients had more frequent attacks and had a tendency for bilateral eye involvement.
It appears that genetic background, radiographic severity of disease, presence of enthesis, and particularly disease duration may be related with uveitis. Although uveitis is usually self-limited, almost 10% of SpA patients may have permanent eye damage.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.