FDA Approves Mepolizumab for Churg-Strauss (EGPA) Save
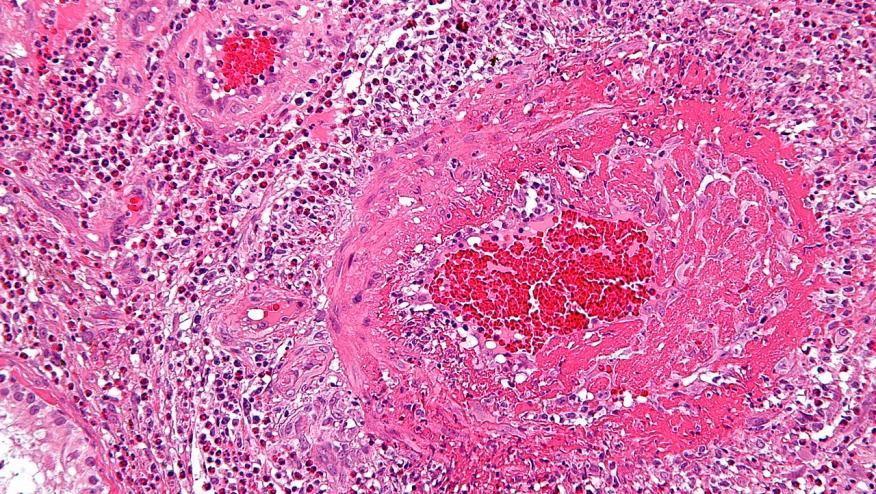
The Food and Drug Administration (FDA) approved use of Nucala (mepolizumab) for use in treating adults with eosinophilic granulomatosis with polyangiitis (EGPA), previously known as Churg-Strauss vasculitis. This is first FDA-approved therapy specifically to treat EGPA.
EGPA is rare with an stimated incidence of 0.11 to 2.66 cases per 1 million per year and an overall prevalence of 10.7 to 14 per 1,000,000 adults.
Nucala, an interleukin-5 antagonist monoclonal antibody, was previously approved in 2015 to treat severe asthma with an eosinophilic phenotype. The current approval occurs because of an FDA priority review through the orphan drug program. Orphan drug designation provides incentives to assist and encourage the development of drugs for rare diseases.
Evidence for the efficacy of mepolizumab was established in a NEJM report (N Engl J Med 2017; 376:1921-1932) published in May 18, 2017. This was a 52 week study of 136 patients with relapsing or refractory EGPA patients (on stable doses of steroids) who were given either 300 mg of mepolizumab or placebo every 4 weeks, for a year. The mepolizumab group had more remissions at both week 36 and week 48 (32% vs. 3%), had a 50% lower annualized relapse rate and more patients in the mepolizumab group (18%) were able to discontinue prednisone.
Although only half the participants treated with mepolizumab had protocol-defined remission, this novel biologic intervention represents a new and significant advance in the treatment of EGPA.
Nucala is administered as 300mg once every four weeks by subcutaneous injection
The most common adverse reactions associated with Nucala in clinical trials included headache, injection site reaction, back pain, and fatigue. There are warnings about the risk of herpes zoster, helminthic infections and avoidance during acute bronchospasm or status asthmaticus.








If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.