FDA Holds Off on Xeljanz Approval for Psoriasis Save
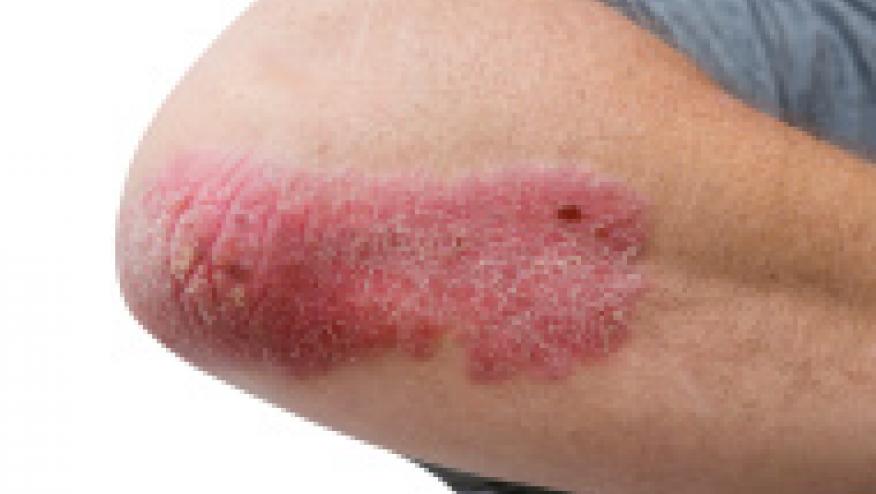
The FDA has denied Pfizer's application seeking an indication for Xeljanz in patients with moderate to severe chronic plaque psoriasis. Instead the FDA has issued a “Complete Response” letter, designed to spell out the specific concerns that the sponsor will need to address should they wish to pursue this indication for Xeljanz in the future.
Xeljanz (tofacitinib), which was approved in 2012 to treat rheumatoid arthritis as an oral alternative to the rising number of injectable biologics, has annual sales of about $500 million. But the agent has met several obstacles, with delayed approval in Canada and non-approval by the EMA for use in the EU.
“Pfizer remains committed to XELJANZ based on the strength of the clinical data for the treatment of psoriasis,” said Kenneth Verburg, PhD, senior vice president and head of global medicines development, Global Innovative Pharma Business. “It is our goal to work closely with the FDA to understand and address their comments about our filing for the use of XELJANZ in patients with chronic plaque psoriasis.”










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.