FDA Panel Recommends Brodalumab Approval in Psoriasis Save
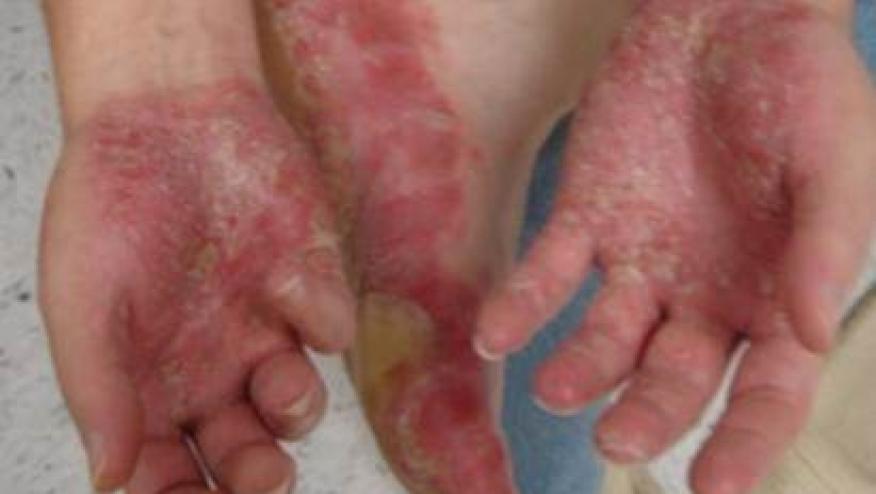
Valeant Pharmaceuticals International's experimental drug to treat psoriasis should be approved as long as certain measures are put in place to mitigate the risk of suicide, an advisory committee to the U.S. Food and Drug Administration concluded on Tuesday.
The FDA is not obliged to follow the advice of its advisory committees but typically does so.
In clinical trials of the drug, brodalumab, there were six suicides across all programs: four in psoriasis studies, one in a rheumatoid arthritis study, and one in a psoriatic arthritis study. Even so, the committee voted 18-0 that the drug should be approved, saying the benefit outweighed the potential risk.
Of those, 14 voted that the drug should only be prescribed alongside a strong risk management program that goes beyond simply including the information in the label. Such programs can include medication guides and communications plans for healthcare providers.
Panelists said there was a need for new drugs for psoriasis and they would like to have brodalumab available as an option. They offered various suggestions about how to mitigate the suicide risk, including a boxed warning and a patient registry to collect patient data and more clearly assess suicide risk.
Some thought the registry should be mandatory and others thought it should be voluntary. Some thought any registry would create unnecessary barriers to accessing the drug and may not reflect a true estimate of the suicide risk.
Valeant itself has a risk management proposal that includes participation in a registry and enhanced communication but no boxed warning.
Brodalumab blocks interleukin-17 to tamp down inflammation. Several other IL-17 inhibitors are already on the market, including Cosentyx (secukinumab) from Novartis AG and Taltz (ixekizumab) from Eli Lilly & Co. The drug would also compete with Amgen's Enbrel (etanercept), Johnson & Johnson's Remicade (infliximab,) and AbbVie's Humira (adalimumab).
About 7.5 million people in the United States suffer from psoriasis, according to the American Academy of Dermatology. The disorder, characterized by raised, scaly skin patches, can be associated with other conditions, including diabetes and heart disease.
Brodalumab was initially developed by AstraZeneca and Amgen. In May 2015, Amgen withdrew from the partnership because of the suicides.
AstraZeneca subsequently licensed global rights to the drug to Valeant, whose fortunes have plummeted over the past year amid criticism of its high drug prices and cloudy relationship with a specialty pharmacy.
This article is brought to RheumNow readers by our friends at MedPage Today. It was originally published on July 20, 2016.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.