Febuxostat Safe in Gout with Moderate-Severe Renal Impairment Save
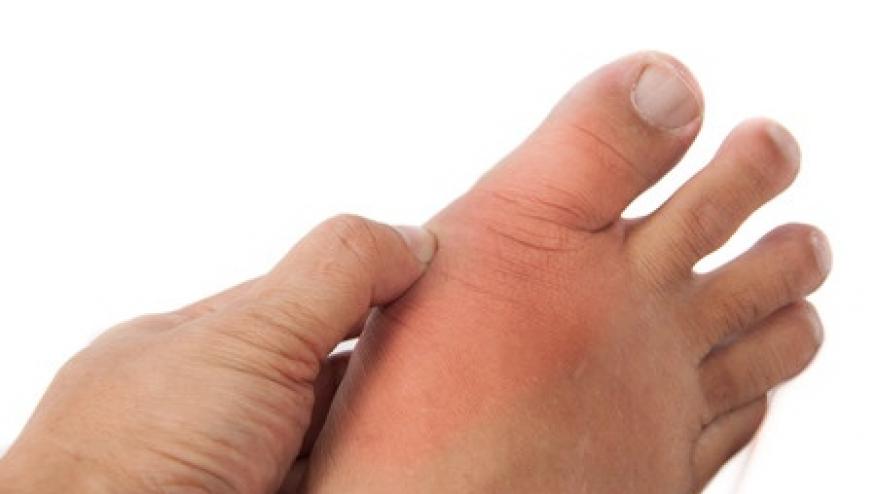
Renal impairment is a risk factor for gout and a barrier to optimal gout management. Many of the drugs used to treat gout require adjustment in those with renal disease.
The product label for febuxostat states that "no dose adjustment is necessary in patients with mild or moderate renal impairment (Clcr 30 to 89 mL/min) and that there are insufficient data on patients with severe renal impairment (Clcr less than 30 mL/min). Hence, caution is advised for such patients.
Renal adverse events reported with febuxostat include hematuria, nephrolithiasis, pollakiuria, proteinuria, renal failure, urgency, incontinence, an tubulointerstitial nephritis.
The safety and efficacy of febuxostat in patients with moderate to severe renal impairment (GFR ≥ 15 to < 50 mL/min) are limited. Saag and colleagues have studied 96 subjects with moderate to severe renal impairment and gout over a 12 month period in a randomized, double-blind, placebo-controlled study using febuxostat 30 mg BID, febuxostat 40/80 mg QD, or placebo.
After 12 month, the change from baseline in serum creatinine was the same in either febuxostat group compared to placebo. However the number with with sUA < 6.0 mg/dL was significantly greater in both febuxostat groups compared to placebo.
Thus, febuxostat is effective and well tolerated in gout subjects with moderate to severe renal impairment. While monitoring is still advised, no initial dose adjustments are necessary.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.