FUTURE 2 Trial Shows Secukinumab to be Effective in Psoriatic Arthritis Save
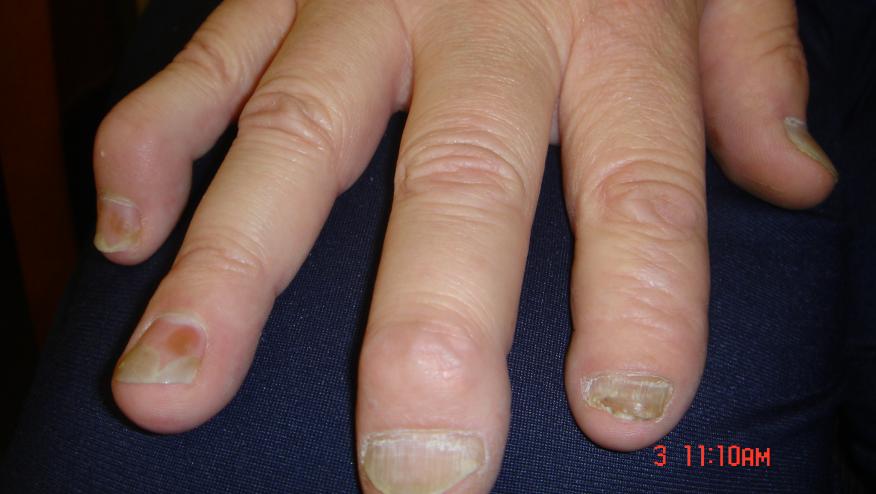
Lancet today published the results of the phase 3, prospecitive, multinational, double-blind, randomized controlled trial of secukinumab (an anti-IL-17A monoclonal antibody) in active psoriatic arthritis. Secukinumab (Cosentyx) is currently approved for use only in severe plaque psoriasis.
In the FUTURE 2 study, 397 PsA patients were randomized to receive secukinumab 300 mg, 150 mg, 75 mg, or placebo weekly 4 time and then every 4 weeks for 24 weeks. A significantly higher proportion of patients achieved an ACR20 at week 24 with secukinumab 300 mg (54%), 150 mg (51%) and 75 mg (29%) compared to placebo (15%). These findings demonstrate the efficacy and safety of secukinumab in patients with PsA.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.