Generic Price Fixing Alleged by State Prosecutors Save
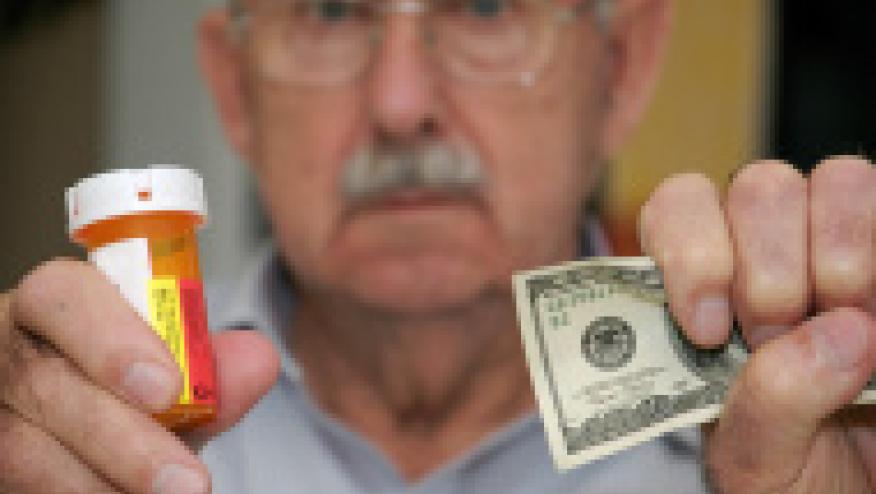
The NY Times and 60 Minutes have reported that Connecticut, along with 43 other states, filed a mega-lawsuit accusing the generic drug makers of a massive, systematic conspiracy to bilk consumers out of billions of dollars. (Citation source: https://buff.ly/2vSmv03)
The lawsuit is based on years of research that claims leading drug companies, including Teva, Pfizer (Greenstone), Novartis, Mylan and Heritage, have prospectively conspired to inflate the prices of generic drugs, by as much as 1,000 percent, to maximize profits.
The industrywide scheme affected the prices of more than 100 generic drugs, according to the complaint, including lamivudine-zidovudine, budesonide, fenofibrate, amphetamine-dextroamphetamine, nystatin, oral antibiotics, blood thinners, cancer drugs, contraceptives and antidepressants.
On 60 Minutes William Tong, the Connecticut Attorney General, states that between 2013 and 2014, a bottle of doxycycline shot up 8,281 percent from $20 to more than $1800. A bottle of asthma medication, albuterol sulfate, jumped more than 4000 percent, from $11 to $434. And pravastatin, a cholesterol drug, up more than 500 percent, from $27 a bottle to $196.
Court documents have laid out the brazen price-fixing scheming of more than a dozen generic drug companies and the executives responsible for sales, marketing and pricing. Despite their efforts to avoid written records of collusion, involved parties coordinated their efforts at at industry meals, parties, golf outings and other networking events. The attorney general of Connecticut says the corrdinated efforts are further documented by phone and text messages, emails, and sales reports. The complaint alleges that these drug companies illegally conspired to increase prices and to thwart competition.
Generic drugs were established by Congress in1984 to create competition and limit drug prices once they are off patent. But recent findings show that 1,215 generics, many of them the most prescribed drugs, jumped on average more than 400 percent in a single year.
Most evidence stems from collusive activity between July 2013 to January 2015, when Teva raised prices on nearly 400 formulations of 112 generic drugs. While Teva Pharmaceuticals appears to be a leader in the conspiracy, numerous companies have latched on to industrywide price increases.
Teva has recently denied any involvement in price fixing.
In February 2018, Teva increased the price of penicillamine to 18,375 (100 pills) to manage Wilson disease and other conditions. Another generic maker, Mylan, was behind the public outrage over the price raising for the life-saving drug EpiPen (from $100 to over $600 for 2 syringes).
The new lawsuit is a more expansive version of a similar suit filed by the previous Connecticut attorney general in December 2016 in the Federal District Court for the Eastern District of Pennsylvania. That suit is currently tied up on court.
The Connecticut AG asserts that "this conspiracy has caused billions and billions of dollars in damages to the people of Connecticut and states across the country. And we're gonna take them on in court and hold them accountable. And they're gonna pay for the money they stole from the American people".







If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.