Hydroxychloroquine Underperforms in a Cutaneous Lupus Trial Save
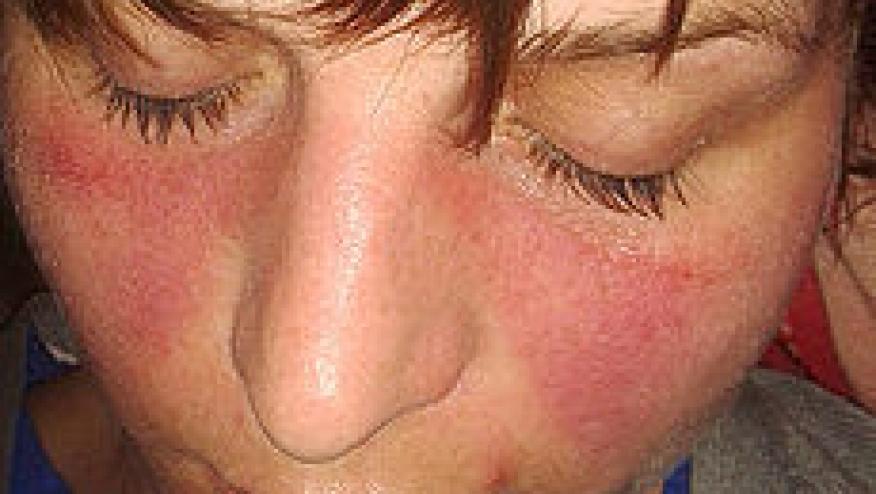
Hydroxychloroquine has become a mainstay in the treatement of patients with systemic lupus erythematosus (SLE) with proven efficacy in managing many domains of lupus, including skin, joint, and other autoimmune features.
Arthritis & Rheumatology has published a a double-blind, randomized, placebo-controlled, parallel-group study of hydroxychloroquine (HCQ) in 103 patients with active cutaneous lupus (CLE) based on an inclusion Cutaneous Lupus Erythematosus Disease Area and Severity Index [CLASI] activity score of ≥4.
Patients received either HCQ or PBO in a 3:1 ratio for 16 weeks and then HCQ for the following 36-week single-blind period. The primary efficacy end point was a reduction in the CLASI activity score at week 16.
The CLASI score at week 16 was significantly improved in both the HCQ and the placebo groups; moreso in the HCQ group (CLASI change −4.6; 95% CI −6.1, −3.1) (P < 0.0001), than the PBO group (change −3.2; 95% CI −5.1, −1.3) (P = 0.002). Yet there was no significant between-group difference (P = 0.197).
Nonetheless, the physicians global assessment demonstrated a greater proportion of “improved” and “remarkably improved” patients in the HCQ group (51.4% versus 8.7%; P = 0.0002 between groups).
While these results show the efficacy and tolerability of HCQ in patients with CLE, they are less than expected. But there are several issues with this Japanese cohort that may explain these marginally effective results. First the patients were older (mean age >40 yrs), had long-standing chronic CLE, 36-46% were on background immunosuppressives (including tacrolimus and dapsone) and over half were active smokers.
Are these reflective of actual practices and real life experiences? Study design and cohort inclusion will usually shape the outcomes of any trial and the clinical question it attempts to answer.








If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.