Intraarticular Capsaicin in Knee Osteoarthritis Save
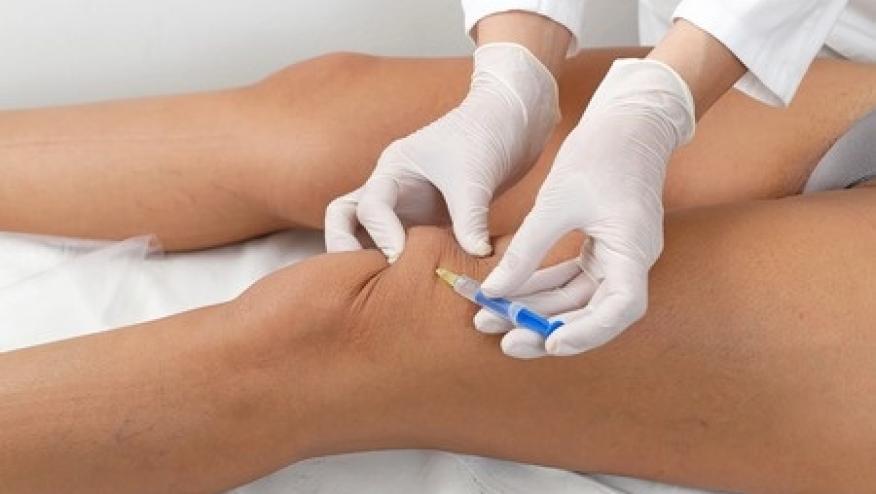
A novel compound, synthetic trans‐capsaicin (CNTX‐4975), has been studied as an intraarticular injection and shown to significantly reduce pain in patients with chronic moderate‐to‐severe osteoarthritis (OA) of the knee.
A phase II, multicenter, double‐blind study enrolled 172 knee OA patients between the ages of 45–80 years. Patients were randomized to receive either intraarticular placebo, or a high‐purity synthetic trans‐capsaicin CNTX‐4975 0.5 mg, or CNTX‐4975 1.0 mg.
A single intraarticular injection (4 ml) of placebo, CNTX‐4975 0.5 mg, or CNTX‐4975 1.0 mg was administered. Premedication with opioid, NSAID, or topical anesthetic (e.g., ethyl chloride, topical or subcutaneous lidocaine), and joint cooling before and after the study injection was permitted.
The primary efficacy end point of the study was improvement in pain as measured by the under the curve (AUC) for change from baseline in daily Western Ontario and McMaster Universities Osteoarthritis (WOMAC) pain Index.
Week 12 results showed superiority for CNTX‐4975 over PBO:
- CNTX-4875 0.5 mg vs PBO for least squares mean difference [LSMD] −0.79, P = 0.0740;
- CNTX-4875 1.0 mg vs PBO LSMD −1.6, P < 0.0001.
- Significant improvements were maintained at week 24 in the 1.0 mg group (LSMD −1.4, P = 0.0002).
Whereas the CNTX-4875 1mg group maintained its lower pain scores (LSMD −1.4; P < 0.001) out to week 24, the CNTX-4875 0.5 mg group lost its pain efficacy after week 12 (LSMD −0.6; P = 0.19).
Acetaminophen use (13,392 mg vs. 21,006mg) and ibuprofen use (7445 mg vs. 9304 mg) at 12 weeks was also significantly less in the intraaritular CNTx-4875 patints compate to placebo treated patients.
Treatment‐emergent adverse events were mild, nonspecific and not significantly dissimilar between groups.
CNTX‐4975 provided dose‐dependent improvement in knee OA–associated pain for 12 to 24 weeks, depending on the dose.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.