J&J Hit with a $247 Million Verdict over Metal-on-Metal Hip Replacements Save
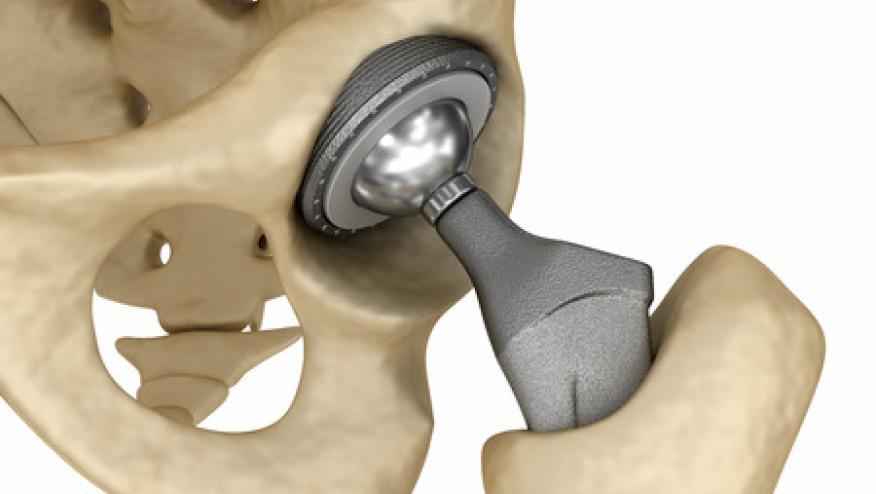
Reuters reports that a federal jury has ordered Johnson & Johnson and its DePuy Orthopaedics division to pay $247 million settlment to six patients who suffered from its defective Pinnacle, metal-on-metal, hip implants. in the US. J&J says it will appeal this decision.
These implants were associated with tissue necrosis, bone erosion and other injuries due to implant design.
J&J won the first Pinnacle test trial in 2014, but since has been held liable. A jury in March 2016 awarded five Texas plaintiffs $500 million in damages. That award was later cut to $150 million. J&J has more than 9,700 such law suits pending.
DePuy ceased selling the metal-on-metal Pinnacle devices in 2013.
Continue Reading
ADD THE FIRST COMMENT
Disclosures
The author has no conflicts of interest to disclose related to this subject







If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.