Lupus Nephritis Therapies Reviewed Save
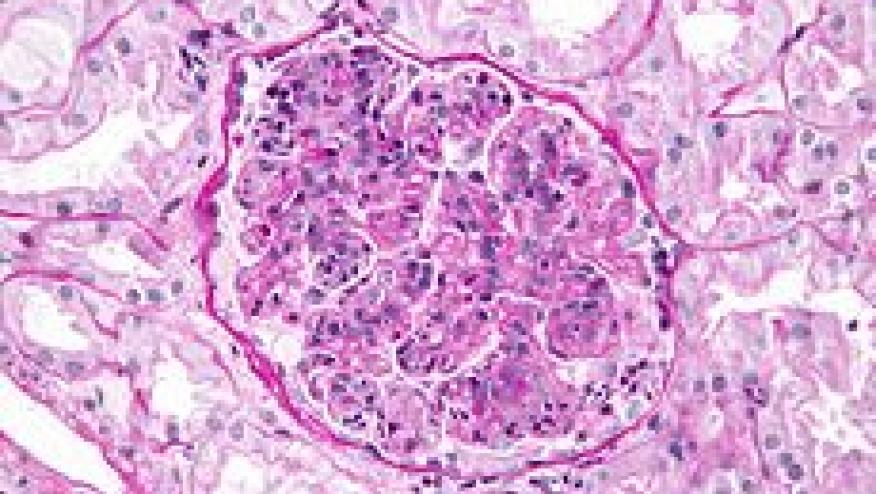
Singh and colleagues have published a systematic review and Bayesian network metaanalyses of clinical trials (RCT) of immunosuppressive drugs and corticosteroids (CS) in patients with lupus nephriti (LN).
Among the 65 RCT that met criteria, they found the following agents to significantly lower risk of endstage renal disease (ESRD) (Odds ratio and 95%confidence intervals):
- cyclophosphamide (CyC; (OR 0.49, 95% CrI 0.25–0.92) or
- CYC + azathioprine (AZA; OR 0.18, 95% CrI 0.05–0.57) compared with standard-dose CS
- high-dose (HD) CYC (OR 0.16, 95% CrI 0.03–0.61) or
- CYC + AZA (OR 0.10, 95% CrI 0.03–0.34) compared with HD CS.
- Conversely, HD CS was associated with higher risk of ESRD compared with CYC (OR 3.59, 95% CrI 1.30–9.86), AZA (OR 2.93, 95% CrI 1.08–8.10), or mycophenolate mofetil (MMF; OR 7.05, 95% CrI 1.66–31.91).
- Compared with CS, a significantly higher proportion of patients had renal response (14 studies) when treated with CYC (OR 1.98, 95% CrI 1.13–3.52), MMF (OR 2.42, 95% CrI 1.27–4.74), or tacrolimus (TAC; OR 4.20, 95% CrI 1.29–13.68).
No differences were noted for the risk of malignancy (15 studies) with these agents.
Compared to CS, a higher risk of herpes zoster (17 studies) was noted for MMF (OR 4.38; (95CI 1.02–23.87), CYC (OR 6.64; 95CI 1.97–25.71), TAC (OR 9.11; 95CI 1.13–70.99), and CYC + AZA (OR 8.46; 95CI 1.99–43.61).
There are numerous effective treatment options for LN, but there is an inherent risk of herpes zoster with these immunosuppressive drugs.





If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.