Monitoring of Novel Therapies in Rheumatology Save
The rheumatology therapeutic landscape has changed almost yearly in the last 15 years, with the introduction of at least 16 biologic or novel agents for the treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), spondyloarthritis (SpA), juvenile arthritis, cryopyrin associated autoinflammatory disease, lupus and gout.
The efficacy of these agents must be weighed against their safety (and cost). The safety of novel therapies should involve: 1) a risk communication strategy; 2) proper patient selection; and 3) a plan for screening and drug safety monitoring.
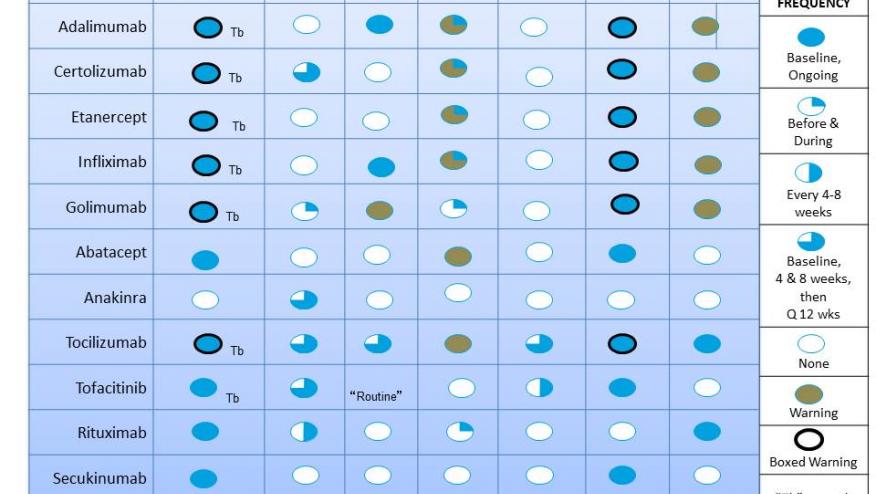
Below is a Table designed to give an overview of the safety recommendations for the 12 drugs used to treat RA, PsA, psoriasis and AS (other biologics and DMARDs are not considered in this report).
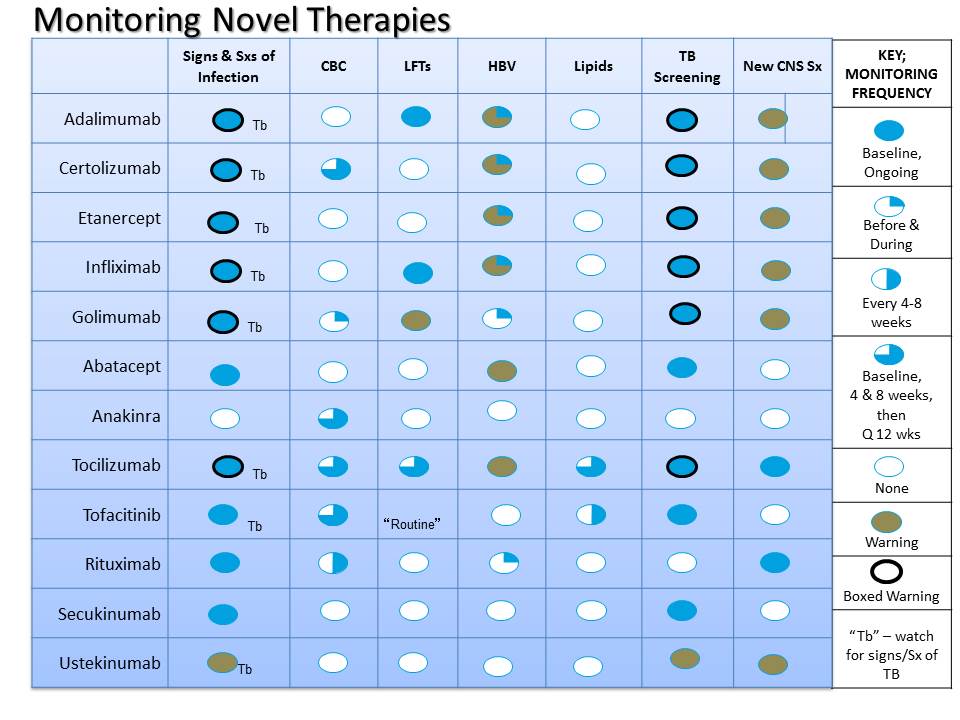
Monitoring for infection: for many of the current agents the Package Insert (PI) states patients should be monitored for signs and symptoms of infection. The only exceptions to this are anakinra and ustekinumab, where such monitoring is not specified. In many (all the TNF inhibitors, tocilizumab), this statement appears in the “boxed warning”. Such monitoring for signs and symptoms requires the physician to a) have a high index of suspicion for infection in these patients and with these drugs and b) ask about infection, fever, or hospitalization. There is no “lab test” to monitor for infection.
TB Monitoring: The PI suggests patients on some agents be monitored for “signs and symptoms of tuberculosis”. Of course the risk of TB is much, much higher in patients on TNF inhibitors and in those born in countries where TB is endemic. While the PI calls for monitoring (and even screening) with tofacitinib, ustekinumab, tocilizumab and screening for TB with the same drugs and abatacept and secukinumab, it should be noted the risk of TB is a lot lower (meaning 10-100 times less frequent) with these agents. The reason for such monitoring or screening is largely based on the way the drug development trials were done. For instance, PPD screening was commonly done in the abatacept and secukinumab trials and thusly appears in the PI. Anakinra, rituximab and apremilast trials did not include PPD and other TB screening, and are therefore not mandated in the PI.
TB Screening: (see above). Is required with all the TNF inhibitors, abatacept, tocilizumab, tofacitinib, secukinumab and ustekinumab. Recognize that if no other TB risk factors are present, the risk of TB is very, very low with abatacept, tocilizumab, tofacitinib, secukinumab and ustekinumab.
CBC/Blood counts: Interestingly, this is required by the PI for some agents, but not others. Largely this has to do with the potential for neutropenia – which is a very infrequent event for most biologics or tofacitinib. The PI recommends CBC monitoring for anakinra, tocilizumab, tofacitinib, rituximab, and certolizumab and golimumab, but not for the other TNF inhibitors (etanercept, adalimumab, infliximab) and abatacept.
Hepatic enzymes: for many rheumatologists, the need for LFT monitoring will be driven by the use of either NSAIDs or DMARDs such as methotrexate (MTX), leflunomide or sulfasalazine. Nevertheless, several agents will require baseline and intermittent hepatic enzyme monitoring which, in the author's opinion, should include AST, ALT, alkaline phosphatase, bilirubin and albumin. The PI calls for LFT monitoring with tocilizumab, tofacitinib, infliximab, golimumab and adalimumab. “Warnings” for hepatic enzyme elevation appear in the PI for the latter two (golimumab and adalimumab). When the FDA reviewed the association of hepatic enzyme elevation with TNF inhibitors, the vast majority of these rare reports were with infliximab, irrespective of MTX use.
Hepatitis B Screening: There are well-reported associations with Hepatitis B reactivation and several of these agents – notably infliximab (an all the TNF inhibitors), abatacept and rituximab. Nevertheless, the PI states patients should be tested before and during for hepatitis B infection. Screening should include tests for HBsAg and HBcAb with or without HBsAb. Hepatitis B is a listed warning for abatacept and tocilizumab.
Lipid Screening: Mechanisms by which control of inflammation with either IL-6 (tocilizumab) or JAK (tofacitinib, baricitinib, etc.) inhibition is unclear. Nevertheless, baseline and intermittent lipid profile testing must be done and should include total cholesterol, LDL, HDL and triglycerides, as all of these may be elevated with tocilizumab or tofacitinib.
CNS Symptom Monitoring: For several agents, the PI calls for the monitoring of signs and symptoms of neurologic disorders, such as multiple sclerosis, optic neuritis, and demyelinating diseases – these are “warnings” for adalimumab, certolizumab and golimumab. There are other unique neurologic conditions associated with other agents. Rituximab patients should be monitored for features of progressive multifocal leukoencephalopathy (PML,) but there is no call for pretreatment or ongoing serologic testing for the JC virus (in my opinion this is not prudent and not cost effective). Tocilizumab has rarely been associated with demyelinating disorders; it is not known if the drug alters the risk or outcome. Ustekinumab has been associated with cases of reversible posterior leukoencephalopathy syndrome (RPLS).
Opportunistic Infections: While not specifically dealt with in the PI, patients receiving these novel or biologic agents may be at some small risk for rare opportunistic infections. None of these infections (histoplasmosis, coccidiodomycosis, BK virus) have reliable or predictive screening serologic tests. Hence the clinician is obliged to a) have a high index of suspicion for rare opportunistic infections (especially if froman endemic area); and b) monitor for signs and symptoms that might indicate an opportunistic infection.
Live Vaccines: nearly all of these drugs caution against the use of live vaccines while receiving a biologic or ustekinumab.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.