Nailfold Videocapillaroscopy in Dermatomyositis Save
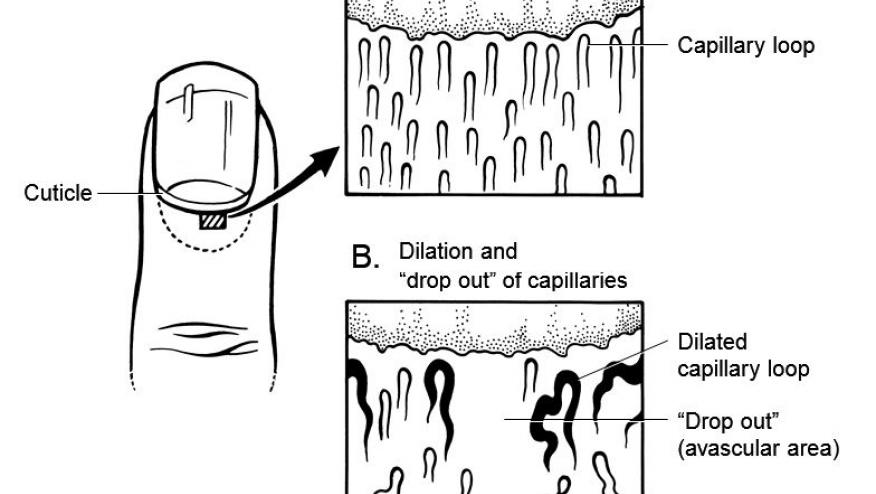
A study of nailfold videocapillaroscopy (NVC) in patients with idiopathic inflammatory myopathies (IIMs) finds abnormalities in more than half of patients suggesting this could be a useful clinical tool in diagnosing and managing patients with IIM.
A prospective study of 70 consecutive Japanese patients with untreated IIMs included clinical assessments, NVC, serologic studies and skin biopsies of DM rash at baseline and 1 year after of immunosuppressive therapy.
NVC abnormalities were seen in 55.7% of IIM patients, moreso in those with dermatomyositis (DM) (65.4%) compared with polymyositis (27.8%) (P = 0.01).
NVC abnormalities were more common in patients with autoantibodies against anti-MDA5 (87.5%) and anti-transcriptional intermediary factor 1γ (TIF-1γ) (88.9%) vs anti-aminoacyl-tRNA synthetase (26.9%, P < 0.001).
NVC positive patients had more severe lymphocytic infiltration in the upper dermis on skin rash biopsy in DM patients P < 0.05).
With 1 year of drug therapy, NVC abnormalities disappeared in 75% of IIM patients, which distinguishes them from scleroderma patients whose NVC changes are static.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.