No Cancer Risk with Systemic Necrotizing Vasculitis Save
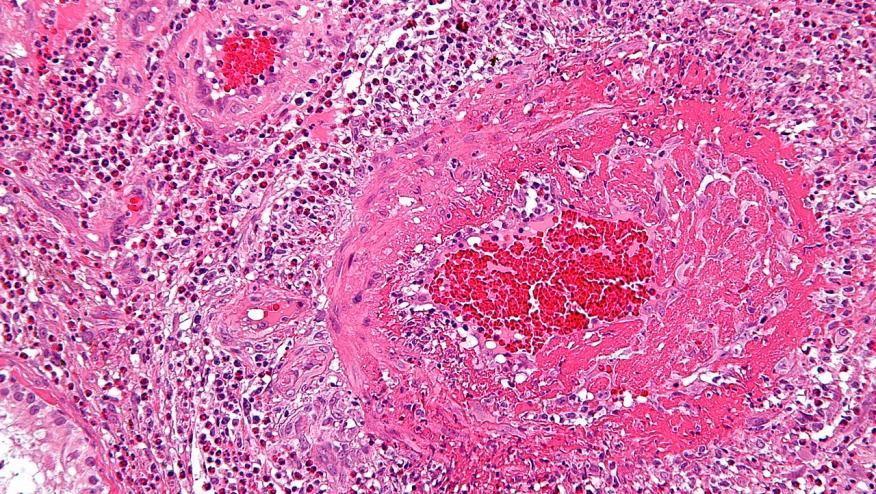
The French Vasculitis Study Group has published that patients with systemic necrotizing vasculitis do not have an increased risk of malignancy; in fact they have a risk that is similar to the general population.
Previous reports have suggested either no risk or a declining risk in the era of better therapies for vasculitis.
The studied 733 SNV patients for a total of 4485.9 person-years (PY) from five randomised controlled trials (CHUSPAN, CHUSPAN 2, WEGENT, CORTAGE and MAINRITSAN) in SNV conducted by the French Vasculitis Study Group. The primary endpoint was the occurrence of malignancy.
All told, there were 39 (5.3%) patients with malignancies (869.5 per 100 000 PY), including solid cancers in 34 (4.6%) cases (757.9 per 100000 PY) and hematological malignancies in 5 (0.7%) cases (111.5 per 100000 PY). The median interval from inclusion to malignancy’s diagnosis was 4.1 years.
The standardized incidence ratios (SIR) for all cancers showed no difference between this cohort and the general population described in the French National registry3 (SIR 0.95 (0.68–1.30); p=0.84).
Risk of malignancy’s occurrence was more likely with:
- Age ≥65 years (HR 2.38 (1.13 to 4.99); p=0.022)
- Azathioprine (HR 3.05 (1.10 to .42); p=0.032) or
- Methotrexate (HR 3.24 (1.07 to 9.81); p=0.038) as maintenance after cyclophosphamided induction
The use of cyclophosphamide induction with rituximab as maintenance therapy was not associated with malignancy (HR 2.65 (0.48 to 14.64); p=0.262).
Malignancy was associated with a poorer survival (median 12.1 years). The cause of death was directly related to the malignancy or its treatment in all 19 (100%) patients with cancer who died.
Whereas the use of conventional immunosuppressive regimens was associated with a higher risk of malignancy, the use of rituximab was not associated with a subsequent malignancy risk. These findings suggest that malignancy should no longer be considered as a predominant feature driving the therapeutic strategy.








If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.