Pilot Data on Rituximab in Henoch-Schonlein Purpura Save
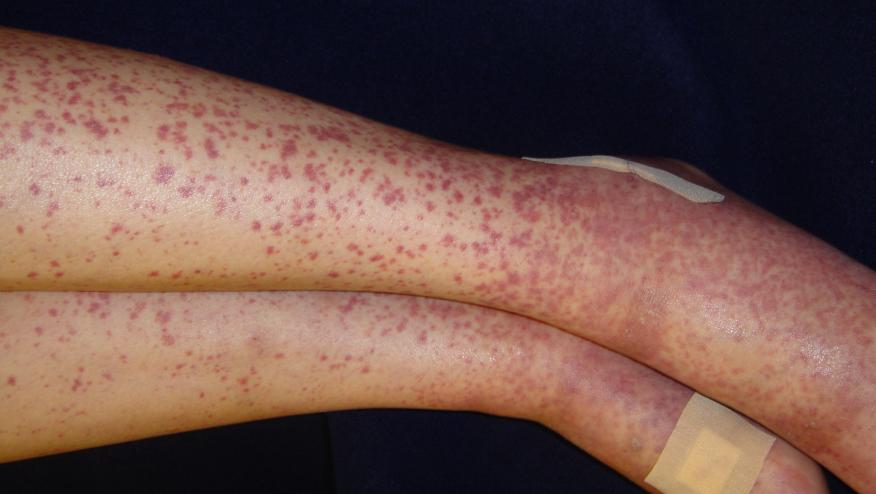
Management of Henoch-Schonlein purpura (HSP) can be challenging as supportive care and avoidance of corticosteroids (CS) are often insufficient options. A new series of case reports and literature review of steroid refractory HSP suggests that rituximab (RTX) may be effective in reducing hospital admissions, overall steroid use, and is capable of inducing remission.
A minority of HSP cases are so severe with either persistent rash, arthritis, abdominal involvement, or renal disease, that CS are needed. Other alternatives for steroid sparing are few.
Crayne et al describes 8 children treated with RTX for chronic refractory HSP. Patients were identified by retrospective review of data between 2006–2014 at a single institution.
Prior to RTX, 7/8 had at least one hospitalization for HSP (median 1.5, range 0–3).
Following RTX, only 2/8 were hospitalized. With RTX use, oral CS use fell significantly from 0.345 mg/kg/day before RTX to 0 mg/kg/day at 6 months (p = 0.078), 1 year (p = 0.0625), and 2 years (p = 0.03) following RTX infusion.
Ultimately 7/8 remission criteria (no active rash, arthritis, nephritis (hematuria or proteinuria), or gastrointestinal involvement).
The authors also describe another eight, mostly single case, reports of RTX use in adults and children with HSP - all with beneficial outcomes.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.