Psoriatic Arthritis Has a New FDA Approved DMARD - Apremilast Save
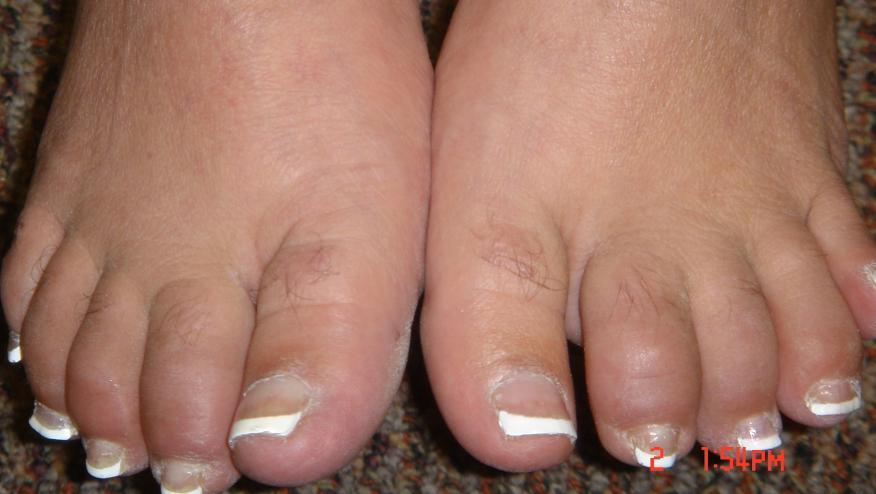
In March 2014 the FDA approved apremilast (Otezla) for use in psoriatic arthritis based on three large"Palace" trials involving 1493 psoriatic arthritis patients. This ACR Hotline reviews the drugs use, efficacy, and toxicity.
Continue Reading








