Rinvoq (upadacitinib) Effective in Psoriatic Arthritis Save
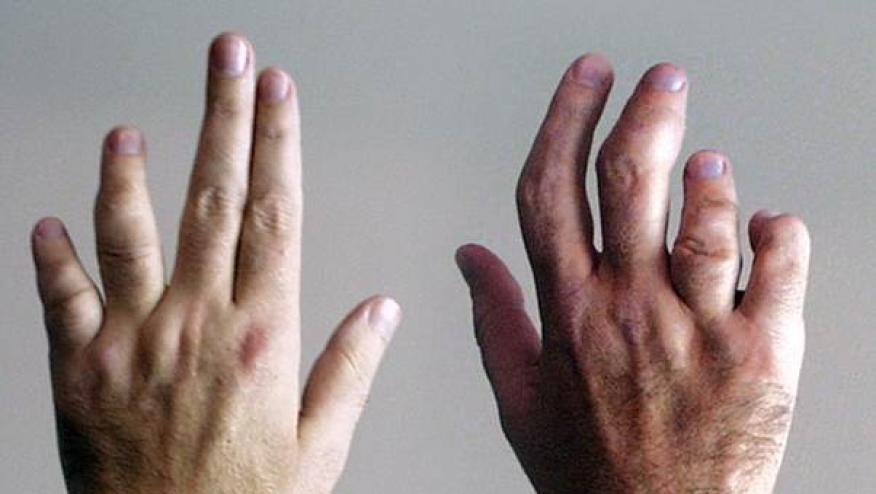
Yesterday, AbbVie reported the top-line study results showing the efficacy of Rinvoq (upadacitinib - UPAD) adult patients with active psoriatic arthritis who previously had an inadequate response to a biologic DMARD.
The SELECT-PsA 2 trial was a Phase 3, multicenter, study of active PsA, not responding to bDMARDs, who were randomized to either placebo (PBO) or UPAD (15 mg or 30 mg) once daily for 24 weeks. The primary endpoint was the number of ACR20 responders at week 12. ACR20 and other responses are shown below:
| Measure | UPAD 15/d | UPAD 30/d | PBO | p Value |
| N = | 211 | 218 | 212 | - |
|
ACR 20 (Wk 12) |
57 | 64 | 24 | p<0.0001 |
|
ACR 50 (Wk 12) |
32 | 38 | 5 | p<0.0001 |
|
ACR 70 (Wk 12) |
9 | 17 | 0.5% | p<0.0001 |
|
PASI 75 (Wk 16) |
52 | 57 | 16 | p<0.0001 |
|
MDA (Wk 24) |
25 | 29 | 3 | p<0.0001 |
The safety profile of RINVOQ was consistent with that of previous studies across indications, with no new safety risks detected. Through week 24, serious infections occurred in 0.5/2.8 percent of patients in the 15/30 mg UPAD groups, respectively, compared to 0.5 percent in the PBO group.
There was one pulmonary embolism reported in the 15 mg UPAD group and none in the 30 mg and placebo groups. There was one non-fatal adjudicated major adverse cardiovascular event (MACE) in the 15 mg UPAD group (acute myocardial infarction) and no MACE in the 30 mg and placebo groups. One death was reported in a patient receiving placebo (motor vehicle accident).
Full results from the SELECT-PsA 2 study will be presented at a future medical meeting.
Rinvoq (upadacitinib) was FDA approved (earlier in 2019) for use in moderately to severely active rheumatoid arthritis.
There are other Phase 3 trials of UPAD in progress in other disorders, including psoriatic arthritis, Crohn's disease, atopic dermatitis, ulcerative colitis, giant cell arteritis, and ankylosing spondylitis.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.