Risk of Gout Onset and Flare Linked to Urate Levels Save
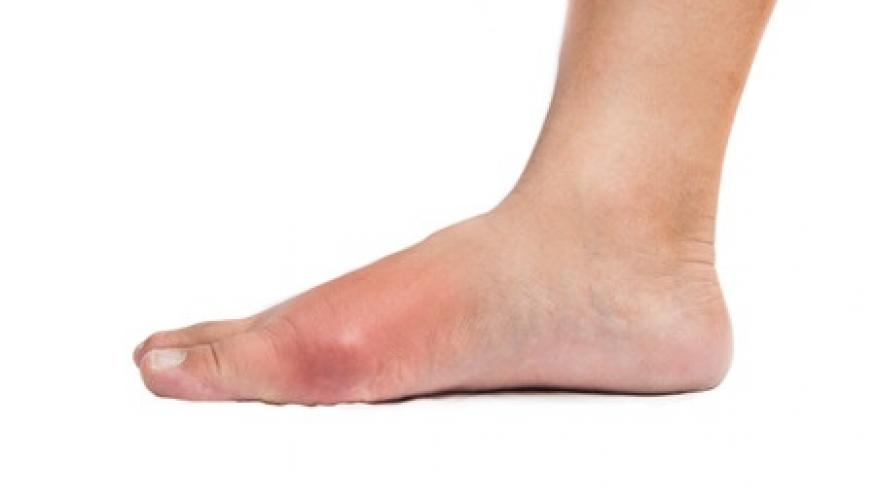
Serum uric acid (SUA) levels are clearly linked to gout. Researchers from Harvard have systemically reviewed the literature to quantify the risk of onset and flare with varying levels of SUA.
Literature review identified 8 eligible articles reporting gout incidence and 18 articles reporting recurrent gout rates in the context of SUA levels.
Gout incidence rates per 1000 person-years from population-based studies ranged from 0.8 (SUA ≤ 6 mg/dl) to 70.2 cases (SUA ≥ 10 mg/dl).
Recurrent gout risk in clinical cohorts ranged from 12% (SUA ≤ 6 mg/dl) to 61% (SUA ≥ 9 mg/dl) among those receiving urate-lowering therapy (ULT), and for those controlled with successful ULT, flare/recurrence rates ranged from 3.7% (SUA 6-7 mg/dl) to 61% (SUA > 9.3 mg/dl).
These data on the risks of higher SUA levels and gout underscores the need to treat to SUA targets as recommended by the American College of Rheumatology and the European League Against Rheumatism.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.