Risk of Psoriasis Complicating TNF Inhibitor Therapy Save
A population-based study of claims data from Korea shows that among inflammatory bowel disease (IBD) patients receiving tumour necrosis factor inhibitors (TNFi) there is a 3.7 per 100 patient-year risk of paradoxically developing psoriasis - a rate that is roughly 3-fold higher than risk in TNFi-naive IBD patients.
Using the Korea National Health Insurance Claim Data between 2007 and 2016, investigators examined all IBD patients (n = 50,502) to compare 5428 TNFi treated patients with 10,856 matched controls who were TNFI naive. Key findings include:
- TNFi patient have increased risk for psoriasis - 36.8 per 10 000 person-years compared to the control group (14.5 per 10 000 person-years) (hazard ratio [HR] 2.357, 95% CI: 1.668-3.331).
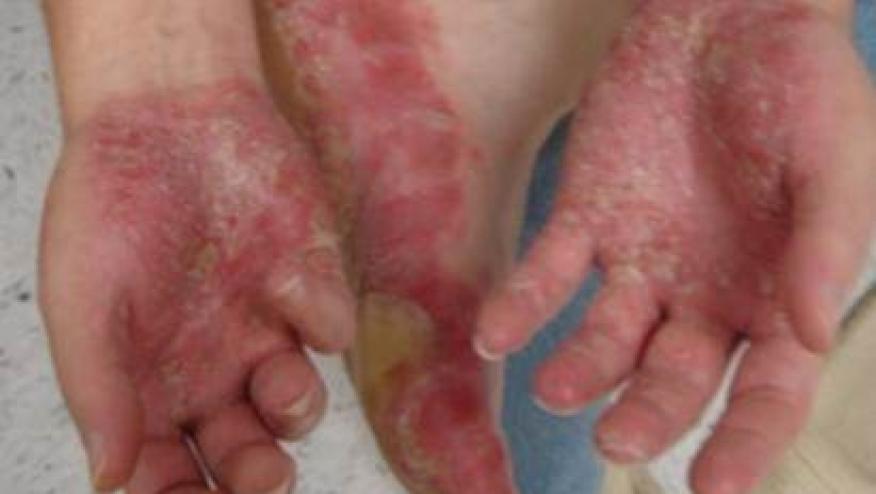
- TNFi treated patients also showed a higher risk of Palmoplantar pustulosis (HR 9.355, 95% CI 2.754-31.780) and psoriatic arthritis (HR 2.926, 95% CI 1.640-5.218)
- Psoriasis risk was roughly the same in Crohn's disease and ulcerative colitis.
- The risk for psoriasiform complications was significant for infliximab (psoriasis: HR 2.6, 95% CI 1.8-3.6; palmoplantar pustulosis: HR 9.5, 95% CI 2.8-32.6,; psoriatic arthritis: HR 3.3, 95% CI 1.8-5.8), but nor for adalimumab.
- Men and younger (10-39 years) patients have significantly higher risks of palmoplantar pustulosis (HR 19.682 [95% CI 3.867-100.169] and HR 14.318 [95% CI 2.915-70.315], respectively).
Mechanisms underlying paradoxical psoriasis are unclear but has been postulated to be due TNFi induction of interferon-a (IFN-a), activation of Th1 and Th17 cells or increased interleukin-10 production.
Psoriasiform diseases may complicate TNFi therapy with the risk of palmoplantar pustulosis being greater in male and younger patients.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.