Rituximab May Halt ILD in Antisynthetase Syndrome Myositis Save
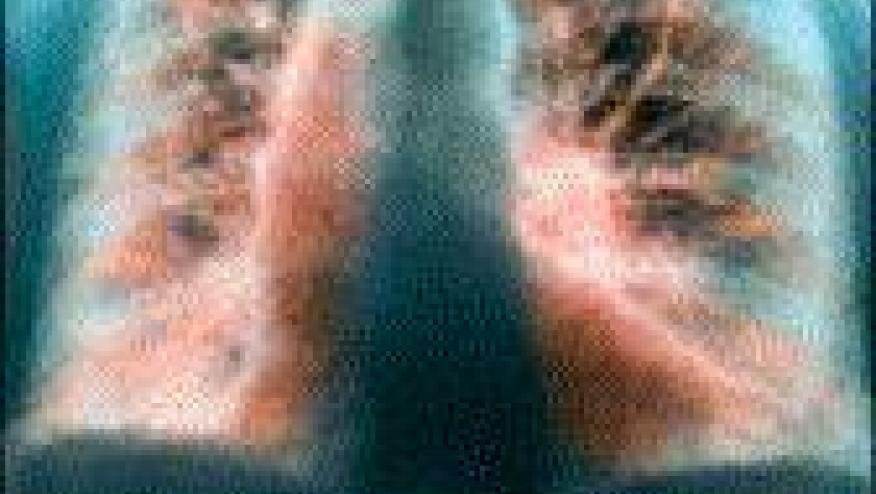
A multicenter study assessed patients with the antisynthetase syndrome (AS) and interstitial lung disease (ILD) and found that rituximab (RTX) therapy was associated with either improved or stable pulmonary outcomes in most.
A retrospective analysis from the University of Pittsburgh and the Brigham and Women’s Hospital pooled their collective experience and analyzed data from 25 patients with AS-ILD, specifically assessing clinical, PFT, and chest CT outcomes using standardized scoring.
All patients had antisynthetase antibodies (16 withJo-1, 6 PL-12, 3 PL-7). In 21/25 (84%), RTX was prescribed for recurrent or progressive ILD. CT findings included CT nonspecific interstitial pneumonia (NSIP; n = 13) and usual interstitial pneumonia (UIP)/fibrotic NSIP (n = 5), and 5 also had cryptogenic organizing pneumonia.
The 12 month outcomes, comparing pre- and post-RTX pulmonary variables, showed improvement in CT score (88%), FVC (79%), total lung capacity (from 56 ± 13 to 64 ± 13) the the glucocorticoid doses decreased from 18 ± 9 to 12 ± 12 mg/day.
DLCO (%) declined slightly at 1 year, but later increased from 42 ± 17 to 70 ± 20 at 3 years.
These uncontrolled and retrospective data provide preliminary evidence of RTX benefit when treating AS-ILD, a condition that is progressive in most patients.








If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.