Severe Cutaneous Sarcoid Treated with Tofacitinib Save
Researchers from Yale have taken a novel approach and shown benefits when using tofacitinib in severe cutaneous sarcoidosis.
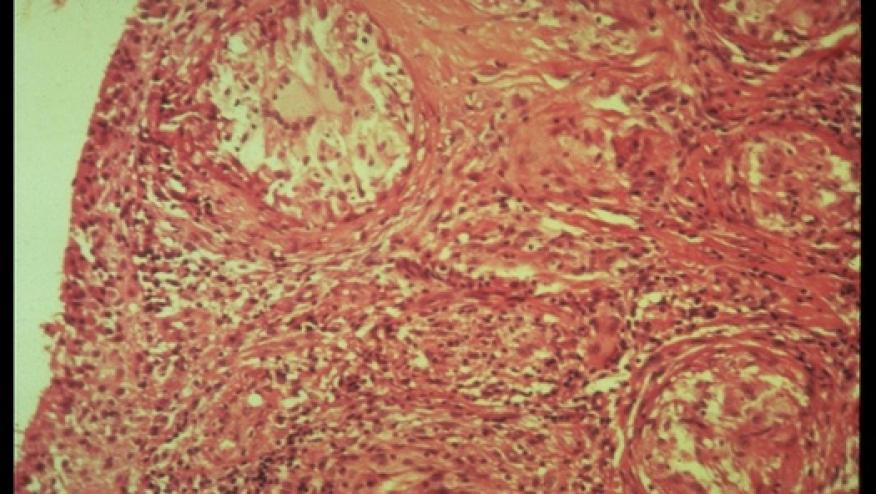
A report in the NEJM describes a 48-year-old female woman who was previously unresponsive to multiple medications and had not received systemic glucocorticoids. She was treated with tofacitinib 10 mg twice-daily and clinically responded with near disappearance of her skin lesions..
There is biologic rationale for this approach as JAK- STAT signaling plays a role in the pathogenesis of sarcoidosis. The authors performed RNA sequencing on biopsied lesions before and during treatment, showing evidence of the Jak-STAT pathway is going from active to inactive. This, coupled with clinical and histologic remission of her skin disease suggests the potential benefit of tofacitinib.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.