Skyrizi Outduels Humira in Psoriasis Save
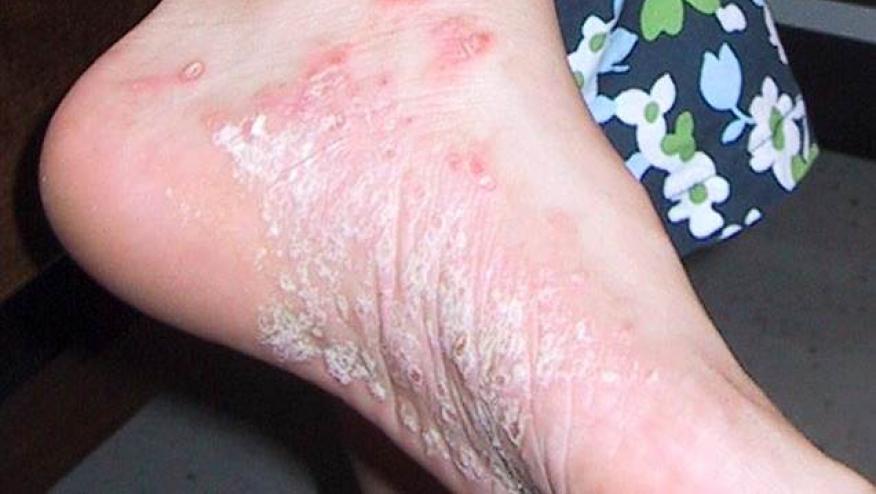
A head-to-head trial has shown that risankizumab was significantly superior to adalimumab in providing skin clearance (PASI90) in patients with moderate-to-severe plaque psoriasis, with no difference in safety signals between the two agents.
Risankizumab (Skyrizi), an interleukin-23 (IL-23) inhibitor, was FDA approved for use in psoriasis in April 2019, becoming the the third IL-23 inhibitor (includes guselkumab [Tremfya] and tildrakizumab [Ilumya]) approved in the last year for plaque psoriasis.
The IMMvent trial was a phase 3, randomised, double-blind, active-comparator-controlled trial that enrolled 605 adults with moderate-to-severe chronic plaque psoriasis and randomized them to either risankizumab (RIS) (150 mg subcutaneously at weeks 0 and 4 ) or adalimumab (ADA) (80 mg subcutaneously at randomisation, then 40 mg at weeks 1, 3, 5, and every other week thereafter during the first 16-weeks of the trial (part A). For weeks 16–44 (part B), adalimumab intermediate responders were re-randomised 1:1 to continue 40 mg adalimumab or switch to 150 mg risankizumab.
Co-primary endpoints in part A were a 90% improvement from baseline (PASI 90) and a static Physician's Global Assessment (sPGA) score of 0 or 1 at week 16.
Nearly all of the RIS (98%) and ADA (96%)patients completed part A (first 16 weeks) of the study.
At week 16, PASI 90 results were:
- RIS - 72%
- ADA - 47% (p<0·0001)
In part B, at week 44, the ADA intermediate responders, achieved a PASI 90 in 66% of RIS treated patients and 21% in those continuing ADA therapy (p<0·0001).
Risankizumab showed significantly greater efficacy than adalimumab in providing skin clearance in moderate-to-severe plaque psoriasis.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.