Skyrizi (risankizumab) FDA Approved for Psoriasis Save
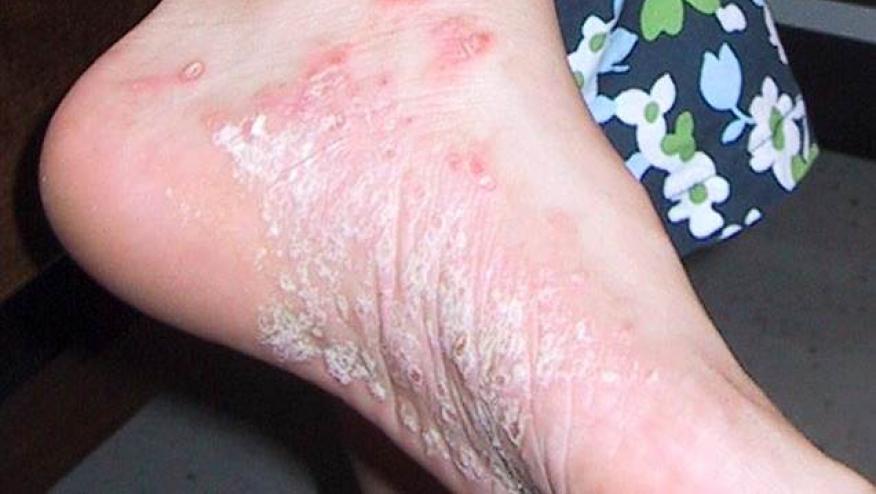
AbbVie has announced that the US FDA has granted the approval of Skyrizi (risankizumab-rzaa) for the treatment of moderate to severe plaque psoriasis.
Skyrizi is an interleukin-23 (IL-23) inhibitor that was also recently approved in Canada and Japan. Skyrizi is the third IL-23 inhibitor (behind guselkumab [Tremfya] and tildrakizumab [Ilumya]) to be approved in the last year.
US approval is based on four large phase 3 pivotal studies: ultIMMa-1, ultIMMa-2, IMMhance and IMMvent. These trials demonstrated 75% PASI 90 scores after 16 weeks of therapy. After 1 year of Skyrizi therapy, over 80% of psoriasis patients achieves a PASI 90 and over half achieve completely clear skin (PASI 100).
Skyrizi is given as two subcutaneous injections ((75 mg. each or 150mg total) initially, and at week 4 and thereafter every 12 weeks.
The most common adverse events were upper respiratory infections (13%), headache (3.5%), fatigue (2.5%), injection site reactions (1.5%) and tinea infections (1.1%). The drug also requires an initial evaluation for tuberculosis (TB) prior to starting therapy.
Abbvie projects Skyrizi to be commercially available in the U.S. in early May 2019.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.