Taltz FDA Approved for Ankylosing Spondylitis (Radiographic Axial SpA) Save
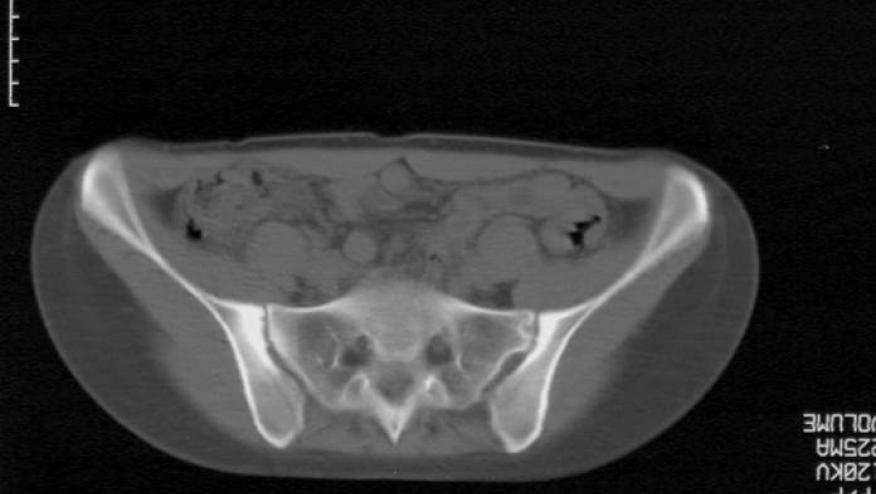
The FDA has approved the IL-17A inhibitor Taltz (ixekizumab) for the treatment of adults with active ankylosing spondylitis (AS: also known as radiographic axial spondyloarthritis).
The recommended dose is 160 mg SC (two 80 mg injections) at Week 0, followed by 80 mg every 4 weeks. The updated package insert can be found here.
According to Lilly, there are 1.6 million people in the US with ankylosing spondylitis, but only 15% of patients with the condition are taking biologic therapies.
Approval is based on the COAST trials in AS. The COAST-V trial studied IXE in biologic naive AS/SpA patients and the COAST-W trial involved patients previously to TNF inhibitors.
In COAST-V, IXE achieved ASAS40 of 48%, compared with 18% of placebo (P .0001) treated patients. ASAS40 response rates in COAST-W were 25% with ixekizumab vs 13% with placebo (P 0.05).
There are no serious "boxed" warnings. Other warnings and precautions include:
- Serious infections
- Tuberculosis (TB): Evaluate for TB prior to initiating treatment
- Hypersensitivity: If a serious allergic reaction occurs, discontinue TALTZ
- Inflammatory Bowel Disease: Crohn’s disease and ulcerative colitis, including exacerbations, occurred in clinical trials
Ixekizumab (IXE) was previously \approved in March 2016 for plaque psoriasis and in December 2017 for psoriatic arthritis.
This will be the second IL-17 targeted biologic (along with Cosentyx) approved by the FDA for ankylosing spondylitis.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.