Tildrakizumab, an IL-23 Inhibitor, is Successful in Plaque Psoriasis Save
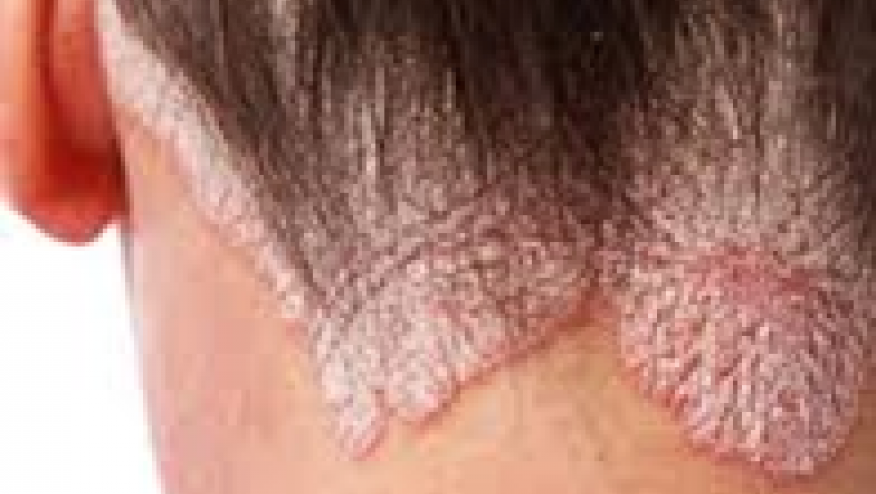
Therapeutic options for cutaneous psoriasis (and psoriatic arthritis as well) are rapidly expanding. Lancet has published the impressive results of two phase III trials of tildrakizumab, a IgG1 antibody against interleukin 23 p19, in patients with active chronic plaque psoriasis.
Two large randomised controlled studies, reSURFACE 1 and reSURFACE 2 enrolled 2062 adults with moderate-to-severe chronic plaque psoriasis with ≥10% body surface area involvement.
In reSURFACE 1, patients received tildrakizumab 200 mg, tildrakizumab 100 mg, or placebo and in reSURFACE 2 they received tildrakizumab 200 mg, tildrakizumab 100 mg, placebo, or etanercept 50 mg given subcutaneously at weeks 0 and 4 during part 1 of the studies and at week 16 during part 2.
In reSURFACE 1, at week 12, a PASI75 was acheived in 62% in the 200 mg group and 64% in the 100 mg group compared with 6% in the placebo group (p<0·0001 vs placebo).
In reSURFACE 2, a PASI75 was seen 66% in the 200 mg group, 61% in the 100 mg group, and 48% in the etanercept group compared with 6% in the placebo group.
Serious adverse events were similar and low in all groups in both trials, with only one death in reSURFACE 2.
These 2 large trials affirm the efficacy of tildrakizumab (an IL-23 inhibitor) in psoriasis and that responses were superior to placebo and etanercept in moderate-to-severe chronic plaque psoriasis patients.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.