TNF Inhibitor and Biologic Induced Psoriasis Save
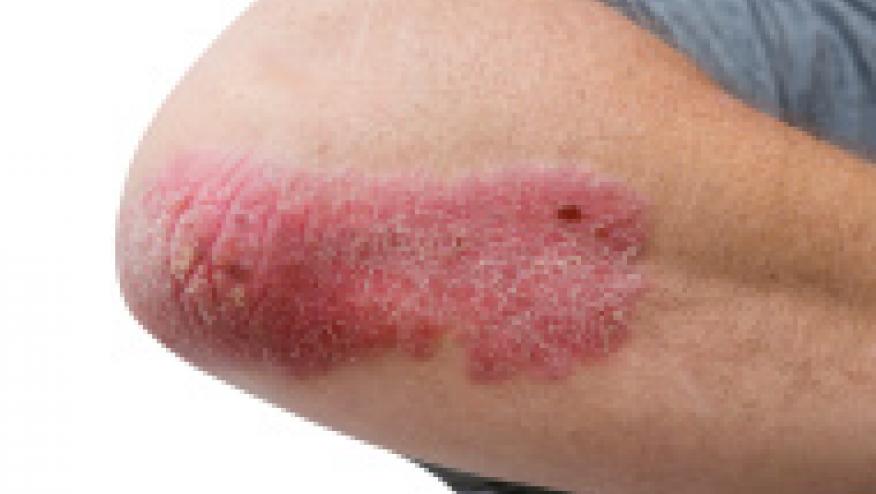
Analysis of RA patients in the German biologics register, RABBIT, shows an increased risk of psoriasis with TNF inhibitor (TNFi) compared to csDMARDs and other non-TNFi biologics.
Among RABBIT RA patients without (n = 14,525) or with a history of psoriasis (n = 375) they assessed the risk of incident psoriasis or flare according to what biologic or csDMARD taken.
Overall, there were 117 incident and 37 recurrent psoriatic events reported. The observed incidence rates for psoriasis:
- TNFi IR = 3.04/1,000 PY
- csDMARDs IR = 0.65/1000 PPY (IRs for abatacept, rituximab and tocilizumab did not differ significantly from csDMARDs.)
Risk of incident psoriasis was higher for TNFi, female sex (HR: 1.7) and smoking (HR: 2.1), but decreased with methotrexate (HR: 0.5). Comedication of TNFi with methotrexate seems to lower the risk of incident psoriasis.
For recurrent psoriasis, IRs for TNFi, abatacept and rituximab were significantly higher than for csDMARDs.
This reported incidence of TNFi induced psoriasis of 3 per 1000 PY is similar to a previously reported large cohort rate of 1 per 1000 PY. Nevertheless, the overall risk is low in RA without psoriasis and rare in those with a history of psoriasis.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.