TNF Inhibitor Therapy and the Risk of Anterior Uveitis Recurrence Save
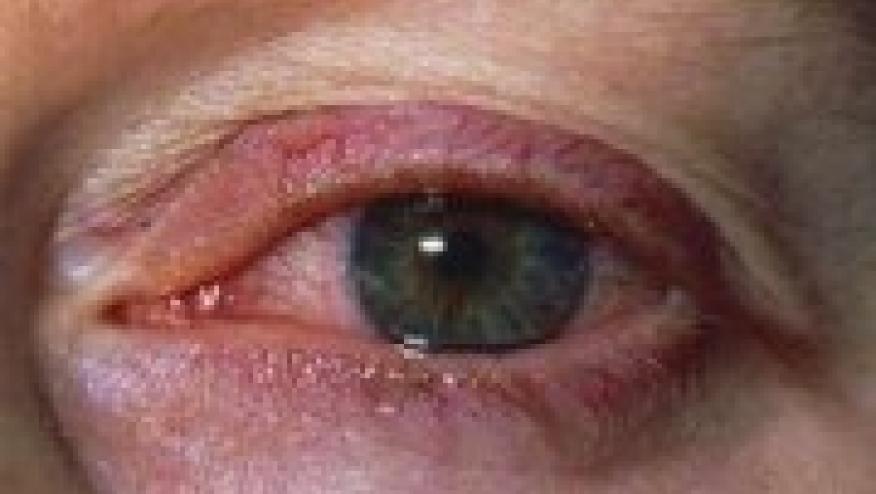
The TNF inhibitor drug development trials have indirectly taught us that monoclonal antibody based TNFi was capable of retarding the recurrence of anterior uveitis (AU) in patients with ankylosing spondylitis (AS).
This issue has been revisited by Swedish investigators who studied the ability of adalimumab (ADA), etanercept (ETN) and infliximab (IFX) on AU recurrence in AS patients using patients from the Swedish Rheumatology Quality Register starting their first TNFi between January 2003 to December 2010.
The study included 1365 patients with AS.
Compared with pretreatment rates, reduced rates for AU rates were seen with ADA and IFX, while an increased AU rate was seen for ETN.
The adjusted HRs for developing AU were significantly higher for ETN versus ADA (HR: 3.86) and ETN versus IFX (HR: 1.99), while the HR for IFX versus ADA was not statistically significant.
These data confirm earlier observational studies showing the efficacy of ADA and IFX in AU. However, this study suggests little or no protection when using ETN. Suggesting mechanistic differences between antibody and receptor based therapies are clinically important in this particular extraspinal manifestation of AS.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.