Tofacitinib Efficacy Revealed in OPAL Study Save
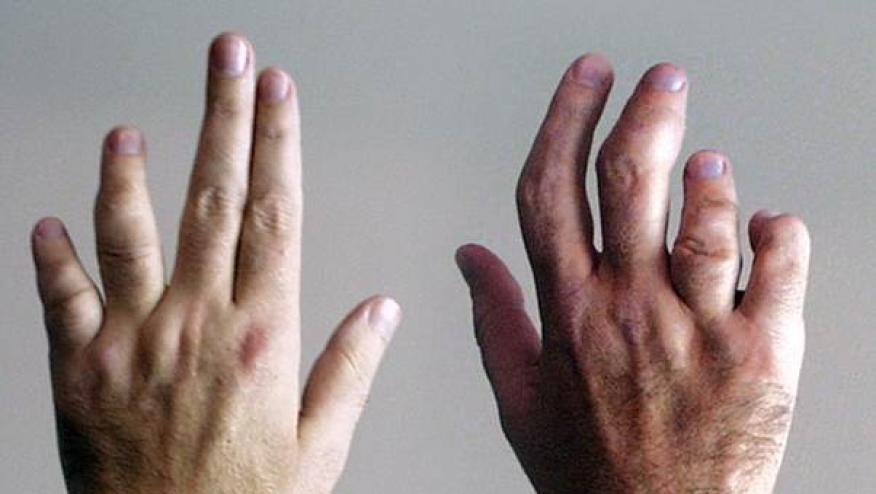
Pfizer has issued a press release of its preliminary results from the OPAL study that examined the efficacy and safety of tofacitinib 5 mg and 10 mg twice daily (BID) in adults active psoriatic arthritis (PsA).
422 PsA patients, on background DMARD therapy, were randomized to receive tofacitinib (5 mg or 10 mg BID), adalimumab 40 mg q2 wk (active control) or placebo in a phase III trial.
The primary efficacy endpoint was met with ACR20 responses for both tofacitinib 5 mg BID and 10 mg BID being superior to placebo at 3 months. Disappointingly, no numeric results were released for comparison and magnitude of benefit.
These presumeably positive results in PsA contrast the less-than-expected results in psoriasis only patients, where recent clinical trials demonstrated that tofacitinib 5 mg bid was inferior to etanercept, but 10 mg bid was equal to etanercept in PASI 75 responses.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.