Treatment Preferences in Still’s Disease Save
In July 2018, RheumNow launched a “Live Vote” survey of US and non-US rheumatologists that asked how they diagnose and treat systemic juvenile idiopathic arthritis (sJIA), also known as “Still’s disease”. The audience was invited by a single email question or social media invite and ultimately addressed four questions about on the referral, diagnosis, pathogenesis and treatment of Still’s disease.
An email invitation was initially sent to over 1400 RheumNow registered rheumatologists. From a total of 351 respondents, 257 these from verified rheumatologists and 162/257 (63%) were from the United States (USA) and the remaining rheumatologists were from 36 countries worldwide. Survey respondents were accrued by direct email in the first week (n = 249) and social media invites in the second week (n = 102).
The following four questions were posed to all. USA and non-USA rheumatologist’s answers were largely similar. Below you can observe how USA rheumatologist responses compare to all rheumatologist responses worldwide.
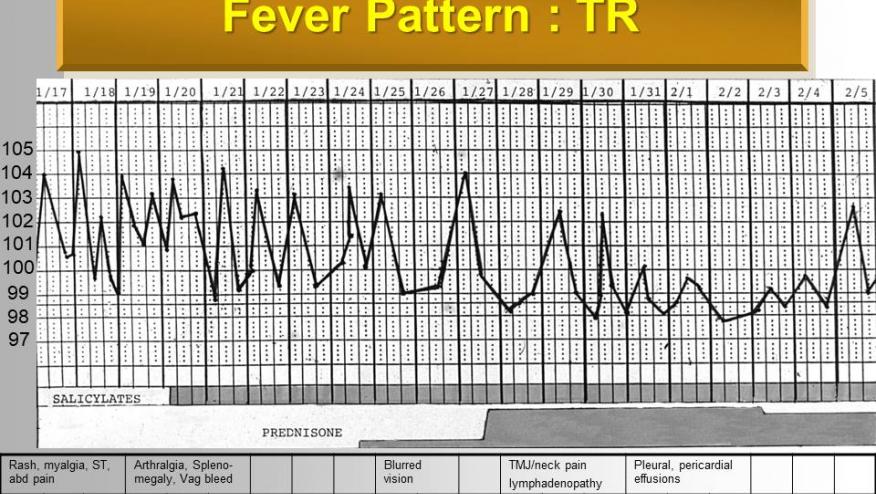
Question 1. Which cytokine do you think is most important in the pathogenesis of Still's Disease (sJIA)? |
||||
|
Response |
All Rheums |
US Rheums |
Commentary |
|
|
a |
IL-1 |
65% |
69.8% |
While nearly 70% of rheumatologists believe Still’s disease is driven by IL-1, one-quarter feel that IL-6 is important in the pathogenesis of sJIA. |
|
b |
IL-6 |
28% |
24.1% |
|
|
c |
IL-18 |
5.1% |
4.3% |
|
|
d |
TNF |
1.9% |
1.9% |
|
Question 2. After steroids, what is your choice of therapy for Still’s Disease? |
||||
|
Response |
All Rheums |
US Rheums |
Commentary |
|
|
a |
MTX |
55.3% |
48% |
After an initial course of steroids, rheumatologists are split on using either MTX or a biologic, with more MTX than biologic use outside the USA. |
|
b |
Biologic |
39.3% |
47.2% |
|
|
c |
Other DMARD |
2.4% |
1.6% |
|
|
d |
Don’t know |
2.9% |
3.2% |
|

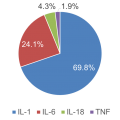
Question 3. Which biologic do you prefer to treat Still’s Disease? |
||||
|
Response |
All Rheums |
US Rheums |
Commentary |
|
|
a |
Anakinra |
67.8% |
71.6% |
In the US and worldwide, nearly 70% of rheumatologists prefer to treat Still’s disease with anakinra (or IL-1 inhibitors). To simplify the survey, we only offered anakinra as a treatment representative of IL-1 inhibition. Tocilizumab is preferred by 15-20% of rheumatologists. Only a minority would prefer to use TNF inhibitors, rituximab or DMARD or other therapies. As such agents have shown limited efficacy in sJIA, it is presumed these agents might be chosen to manage the articular disease more so than the systemic manifestations of sJIA. |
|
b |
Tocilizumab |
19.5% |
15% |
|
|
c |
TNF inhibitor |
4.9% |
6.3% |
|
|
d |
Rituximab |
1.5% |
3.2% |
|
|
e |
I prefer a DMARD |
2.9% |
1.6% |
|
|
f |
Other |
3.4% |
4.7% |
|
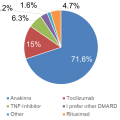
Question 4. Who referred your last Still’s patient to you? |
||||
|
Response |
All Rheums |
US Rheums |
Commentary |
|
|
a |
Pediatric rheumatologist |
3.5% |
1.6% |
The responses to this question indicate that sJIA and adult-onset Still’s disease patients are referred to the rheumatologist for care infrequently from multiple channels and clinical practitioners. Surprisingly, 15-19% of rheumatologists worldwide are potentially inexperienced or do not care for patients with this rare disorder, Still’s disease. The majority of referrals come from outside of rheumatology practices – with PCPs, hospitalists and infectious disease referrals accounting for nearly 60% of all cases seen by the rheumatologist. Roughly 10% of patients come from other (pediatric or adult) rheumatologists and another 9% are self-referred. |
|
b |
Adult rheumatologist |
10.3% |
10.4% |
|
|
c |
Primary care physician (PCP) |
27.1% |
29.6% |
|
|
d |
Infectious disease MD |
6.4% |
4.8% |
|
|
e |
Hospital physician |
28.6% |
25.6% |
|
|
f |
I don’t have any Still’s patients |
14.8% |
19.2% |
|
|
g |
Self-referred |
9.4% |
8.8% |
|
This survey was undertaken to ascertain how rheumatologists consider, diagnose and manage sJIA patients. This is a significant issue as many are unaware of existing criteria for the diagnosis of sJIA and the current treatment options for sJIA and numerous, including corticosteroids, disease modifying ant rheumatic drugs (DMARDs), interleukin-1 (IL-1) or interleukin-6 (IL-6) inhibitors.
Currently, only tocilizumab and canakinumab are approved by the U.S. Food and Drug administration for use in active systemic onset JIA patients 2 years of age and older. Based on this sampling, it appears that many prefer to start therapy with an IL-1 inhibitor, usually after a course of steroids and sometimes after a trial of MTX or other DMARD.
These findings suggest there are significant unmet needs in the diagnosis and management of sJIA patients. More so, recognizing that those who initially diagnose or consider the diagnosis of sJIA are non-rheumatologists.
It is also unclear from this survey whether the optimal treatment algorithm should include DMARDs or should begin with early biologic therapy, and whether initial therapy with an IL-1 inhibitor is preferred over IL-6 inhibition or vice versa.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.