Two-Fold Increase of Demyelinating Diseases with TNF Inhibition Save
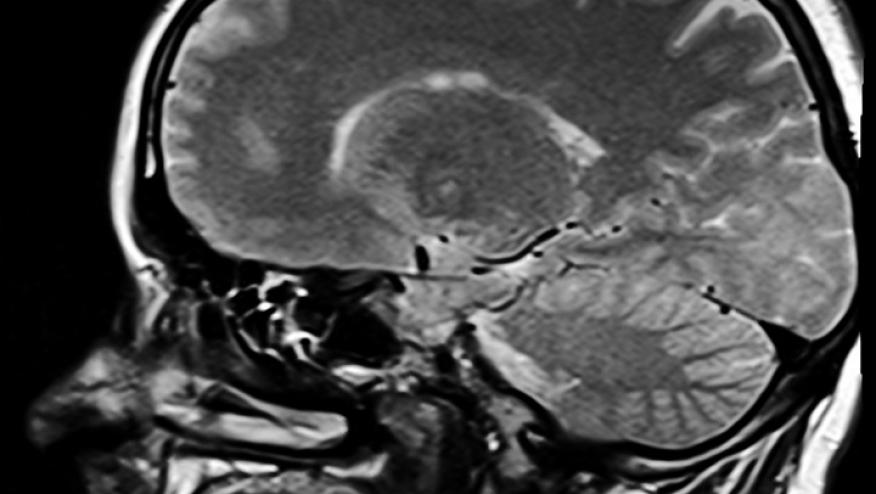
Despite the potential contributory role of TNF in the pathogenesis of multiple sclerosis, several trials have shown that TNF inhibitor (TNFi) use may lead to worsening of MS, optic neuritis and other demyelinating disorders. This particular safety warning has been in the product label for nearly 15 years, yet little is known about why this happens, how often or what the scope of potentially inducible neurologic disease may be.
Researchers in Denmark investigated this potential association between demyelinating disease and inflammatory bowel disease (IBD) and those receiving TNFi for their IBD.
Using a national population-based cohort, they compared rates of central demyelinating diseases among patients with IBD exposed and unexposed to anti-TNF to data drawn from the Danish Civil Registration System, between January 1, 1999, to December 31, 2012.
The neurologic outcomes of interest were defined as a diagnosis of a central demyelinating disease, which includes multiple sclerosis, optic neuritis, transverse myelitis, and other central demyelinating diseases. Patients with a history of demyelinating diseases and those taking TNFi prior to 1999 or prior to IBD diagnosis were excluded.
Among 54843 IBD patients, 4504 received TNFi and were compared to 16,429 unexposed patients. IBD patients were roughly 40 yrs. of age with a mean disease duration 4.0 years.
When comparing TNFi exposed to unexposed (controls) patients, there were 11 demyelinating events in the exposed and 17 demyelinating events in the unexposed controls. Thus, higher rates were seen in the exposed vs. unexposed (7.5 events per 10000 person-years [95% CI, 4.1-13.5] vs. 3.3 events per 10000 person-years [95% CI, 2.1-5.4]).
Exposed patients with demyelinating disease had 2 cases of multiple sclerosis; 5 optic neuritis; and 4 other central demyelinating diseases. Unexposed patients had 5 multiple sclerosis; 6 optic neuritis; 1 transverse myelitis; 5 other central demyelinating diseases.
Overall, there was a two-fold increased risk of central demyelinating disease when using a TNFi in patients with IBD (HR 2.19 [95% CI, 1.02- 4.71]).
These findings support the potential association and the practice of avoiding TNFi in patients with MS, optic neuritis or other demyelinating disorders. There is little evidence that TNFi worsens or affects other degenerative or inflammatory neurologic disorders.








If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.