Uveitis Events Reduced with Select TNF Inhibitors Save
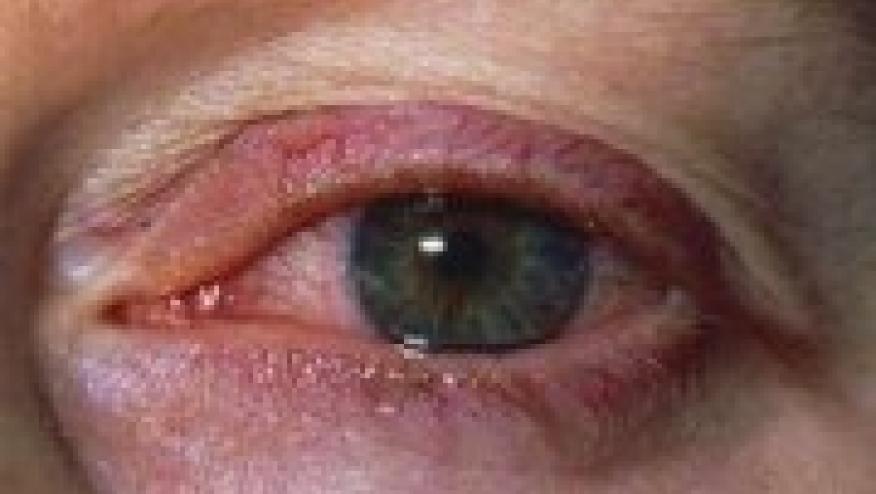
Uveitis may occur in up to 40% of spondyloarthritis patients. Metanalyses have shown that treatment with tumour necrosis factor-α inhibitor (TNFi) may reduce the rates of anterior uveitis (AU). A multicenter study from Sweden and Norway has confirmed that amongst TNFi, adalimumab (ADA) and infliximab (IFX) offer better protection against AU than etanercept (ETN).
Real world data was extracted from the Swedish Rheumatology Quality Register and examined ankylosing spondylitis patients from multiple centers starting ADA, ETN or IFX as their first TNFi between 2003 - 2010. The study included 1365 ankylosing spondylitis patients (406 ADA, 354 ETN, 605 IFX), and they examined AU rates prior to and after TNFi initiation.
AU rates with ADA and IFX therapy were decreased, while ETN was associated with an increase compared to pretreatment rates. The adjusted HRs for AU on ETN were higher compared to ADA and IFX (ETN HR: 3.86 95% CI 1.85 to 8.06; IFX HR: 1.99, 95% CI 1.23 to 3.22). There were no differences in rates seen between IFX and ADA.
These real world data confirm prior collective observations suggesting that monoclonal antibody based TNFi (ADA, IFX) have a clear advantage over ETN in preventing uveitis.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.